Purpose
Conservation and recovery of nitrogen (N) and phosphorus (P) from livestock, industrial, and municipal effluents are important for economic and environmental reasons. Therefore, a need exists for improved systems and methods for N and P recovery from wastewater, especially by using fewer chemicals. A new method was developed using electrochemistry to enhance the gasification and rate of ammonia capture by a gas-permeable membrane and the solubilization and the rate of phosphate capture using P-precipitating compounds. The process was tested using liquid swine manure. It recovered 86% of the ammonia and more than 93% of the phosphorus contained in the manure.
What Did We Do?
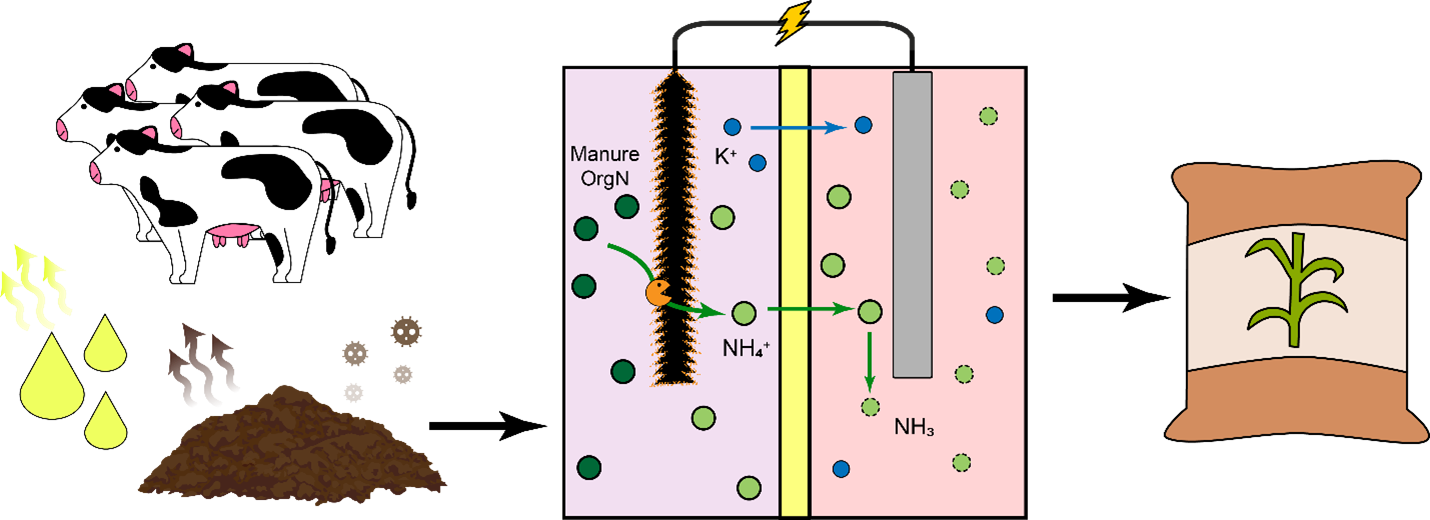
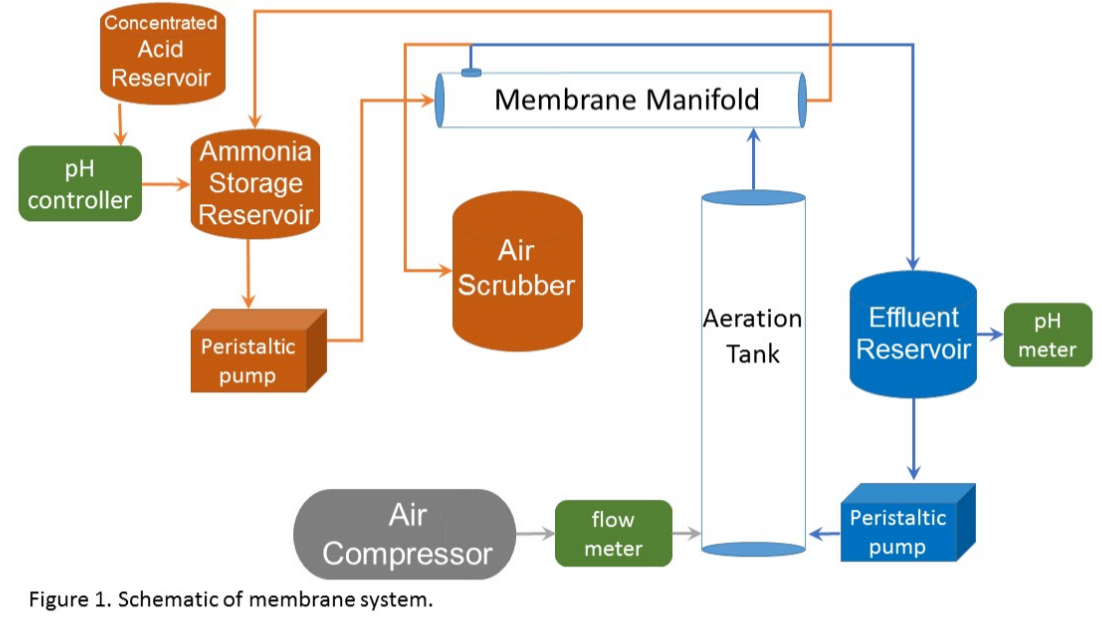
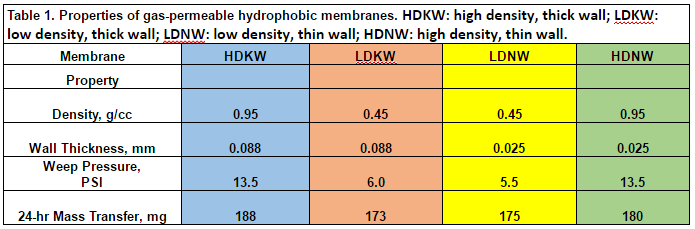
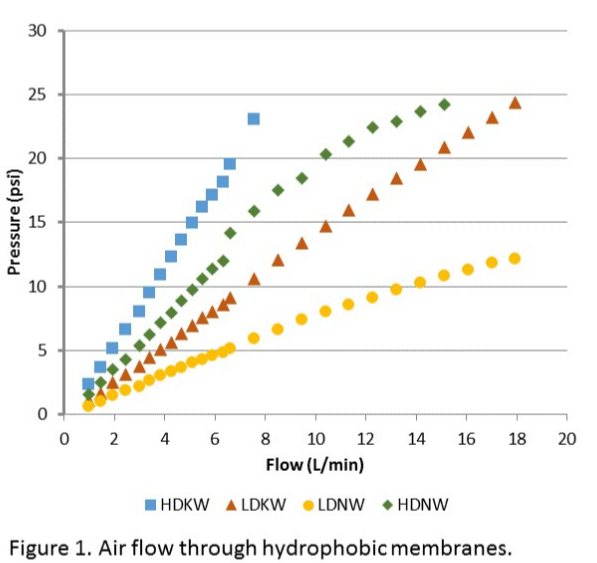
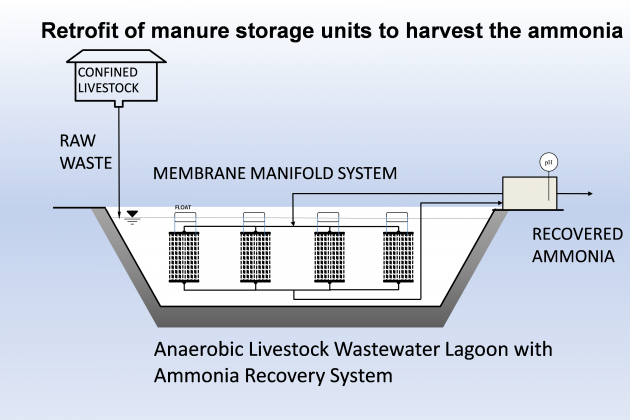
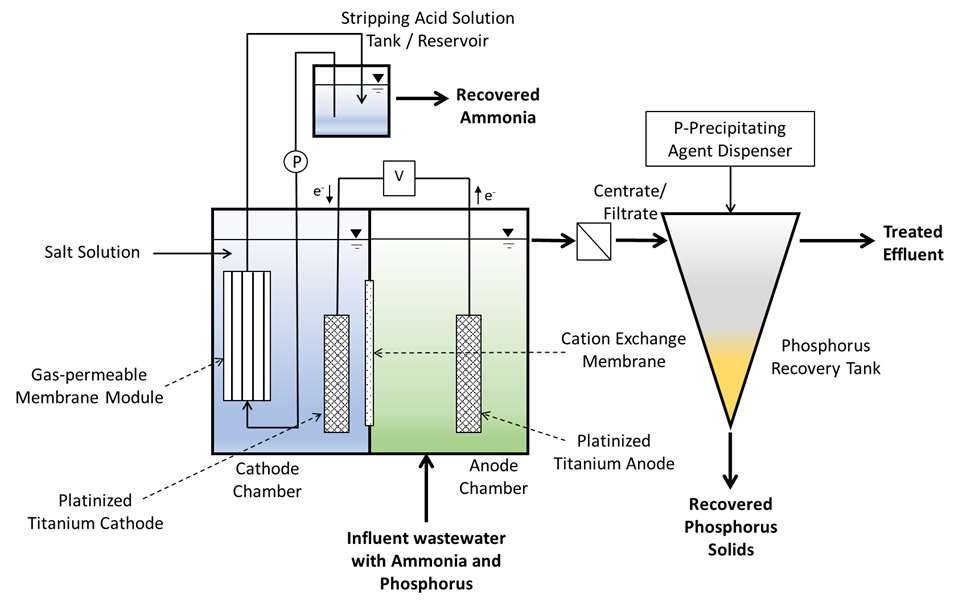
This work aimed to develop new technology for simultaneous N and P recovery that eliminates alkali chemicals used to increase pH for quick N capture using gas-permeable membranes (Vanotti and Szogi, 2015), and also eliminates acid chemicals used to solubilize the P in the manure before precipitation with P-precipitating agents (Szogi et al., 2018). The new N and P recovery system used in this example is described by Vanotti et al., 2024. It has a cathode chamber, an anode chamber, a stripping acid solution tank, and a phosphorus recovery tank (Fig. 1). The cathode chamber is fitted with a gas-permeable membrane manifold. The cathode chamber is fitted with a gas-permeable membrane manifold and contains a salt solution. The wastewater containing ammonia and phosphorus is pumped into the anode chamber. The ammonium (NH4) in the anode chamber permeates into the cathode chamber through a cation exchange membrane placed between chambers. The cathode increases the pH of the liquid and accelerates the rate of passage of ammonia through the gas-permeable membrane into an acid-stripping solution contained in a stripping tank/ reservoir and recirculated through the membrane manifold in a closed loop. The wastewater in the anode chamber is acidified by H+ released by electrolysis in the anode. The anode chamber effluent, with most of the P solubilized, is passed through a centrifuge or filter to separate suspended solids without phosphorus and liquid filtrate/centrate with phosphate. Phosphorus precipitating compounds used were MgCl2 and Ca(OH)2. After rapid mixing, the phosphorus precipitates as a solid. This precipitation proceeds quickly as a result of the previous removal of the carbonate alkalinity in the anode chamber, which interferes with phosphate precipitation.
What Have We Learned?
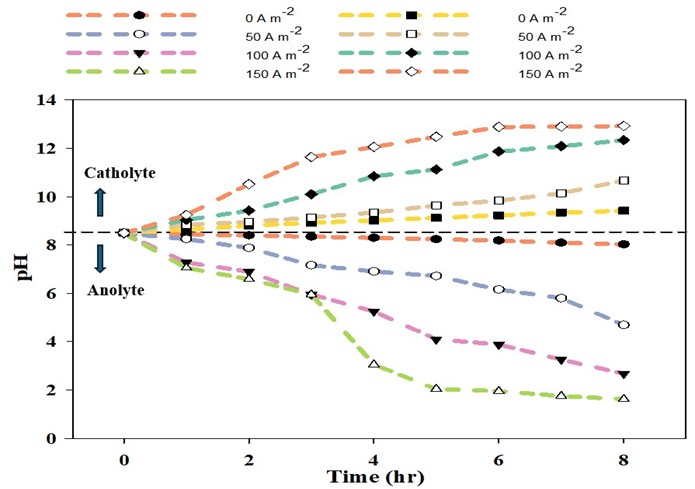
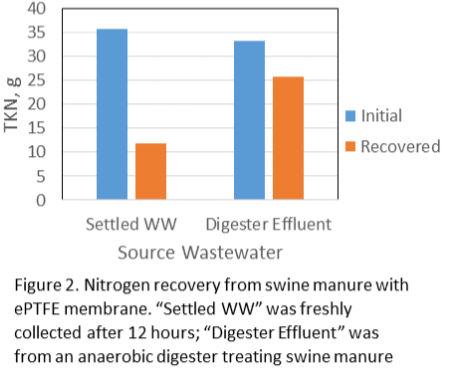
In tests with liquid swine manure, the pH in the cathode chamber was increased due to the electrochemical production of OH-, from 5.8 to 12.5 (Fig. 2). The wastewater’s ammonia was removed from the anode chamber and recovered in the stripping acid solution with 86% recovery efficiency (Fig. 3).
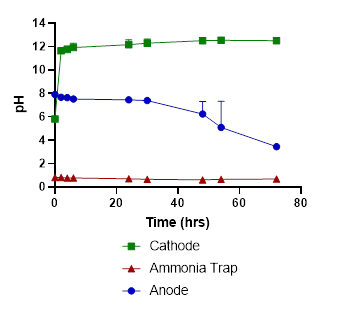
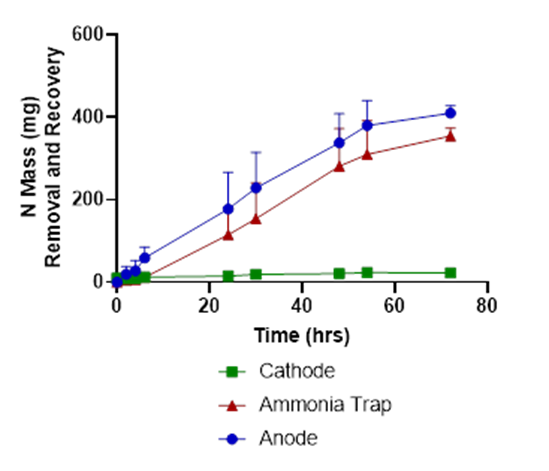
The wastewater pH in the anode dropped from 7.9 to 3.5, and carbonate alkalinity dropped from 10750 mg/L to 0 mg/L (Figures 2 & 4). The acid was produced by oxidation at the anode (2 H2O → O2 + 4 H+). These conditions transformed the P from manure particles into soluble phosphates that were efficiently recovered in the phosphorus recovery tank. For example, using the P-precipitating compound Ca(OH)2, the process recovered 93% of the total P in a P precipitate solid compared to only 4.6% in a control without electrochemical treatment (Fig. 5). Using the P-precipitating compound MgCl2, the process recovered 95% of the total P in a P precipitate solid compared to only 6% P recovery in a control without electrochemical treatment (Fig. 5).


Future Plans
USDA-ARS seeks a commercial partner to bring this technology to market. For more information on commercialization, contact: Mrs. Tanaga Boozer, Technology Transfer Coordinator, USDA-ARS, OTT Southeast Area, tanaga.boozer@usda.gov
Authors
Presenting & corresponding author
Matias Vanotti, USDA-ARS, Matias.vanotti@usda.gov
Additional authors
M.B. Vanotti, A.A. Szogi, P.W. Brigman, and S. Rawal, United States Department of Agriculture (USDA), Agricultural Research Service (ARS), Coastal Plains Soil, Water and Plant Research Center, Florence, South Carolina.
Additional Information
Szogi A.A., Vanotti, M.B., Shumaker, P.D. 2018. Economic recovery of calcium phosphates from swine lagoon sludge using Quick Wash process and geotextile filtration. Frontiers in Sustainable Food Systems 2, 37, https://doi.org/10.3389/fsufs.2018.00037.
Vanotti, M.B., and Szogi, A.A. 2015. Systems and methods for reducing ammonia emissions from liquid effluents and recovering ammonia. U.S. Patent 9,005,333 B1. U.S. Patent and Trademark Office.
Vanotti, M.B., Szogi, A.A., Brigman, P.W., and Rawal, S. 2024. Systems for treating wastewater using electrochemistry. U.S. Patent Appl. 18/808,123. U.S. Patent and Trademark Office
Acknowledgements
This research was part of USDA-ARS National Program 212, ARS Project 6082-12630-001-00D. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2025. Title of presentation. Waste to Worth. Boise, ID. April 7–11, 2025. URL of this page. Accessed on: today’s date.