![]() Waste to Worth home | More proceedings….
Waste to Worth home | More proceedings….
Why Capture Ammonia Released from Animal Manure?
Excessive emissions of ammonia (NH3 ) from animal manure negatively impact the environment with potential to pollute air, soil and water, and produce malodors. The objective of this study was to assess NH3 mitigation from liquid dairy manure (LM) using tubular acid-filled gas-permeable membranes (GPM) in laboratory experiments; and, to evaluate the possibility of scaling up the NH3 mitigation system for use on AFOs.

Fig 1. Schematic diagram of NH3 capture and recovery set-up in laboratory experiments |
What Did We Do?
Initially, a bench-scale study of NH3 capture and recovery system from LM using a sulfuric acid-filled (pH=0.36) tubular GPM system was conducted (Fig .1). Four LM chambers with different surface areas were used with a constant depth of LM in each chamber to investigate the effects of surface areas on NH3 diffusion through membrane. Then the acid was diluted to pH of 2 and higher and the experiments were repeated by using one chamber to assess how diluted acid may extract NH3 from LM. For improving the mitigation process, a pH controller and acid dosing system (Fig. 2) was used to keep the pH of diluted acid at a desired level. To test the performance of the scaled-up system under field condition (Fig. 3) a prototype of the optimized laboratory NH3 mitiagation system was constructed and run in a dairy lagoon. In all experiments, real time NH3 and pH measurements were made from acid solution and LM to compare extraction and recovery of NH3 under laboratory and field conditions.
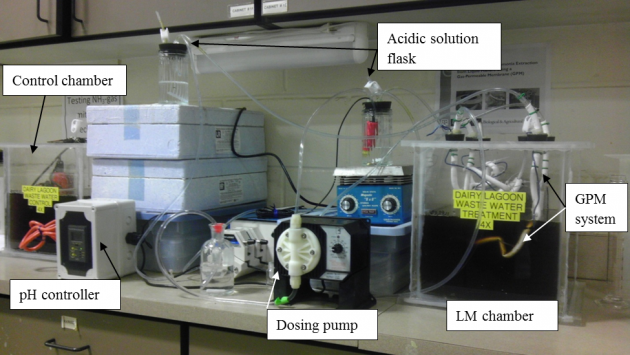
Fig 2. Acid pH controller and acid dosing pump for improving NH3 mitigation system |
What Have We Learned?
Laboratory studies showed that two GPM systems, one submerged below the LM surface and the other suspended above the LM surface, resulted in nearly 50% removal (diffusion) of NH3 from the LM in less than 20 days. Ammonia was captured in concentrated sulfuric acid (pH=0.36) as ammonium sulfate solution (by-product). The GPM system was capable of removing NH3 from the air above (headspace) the LM. Moreover, diluted sulfuric acid with pH 2 or higher could also extract NH3 from LM. Application of diluted acid was essential to decrease the risk of handling strong acids. Also, the automatic pH controlling and acid dosing system increased the efficiency of concentrating NH3 in the acid by about 50%. Doubling the flow rate of acid circulation in the GPM system increased the concentration of by-product by 10%. A pilot scale of the GPM mitigation system in a dairy lagoon showed its feasible to harvest NH3 from LM under field condition (Fig. 3).

Fig 3. Field-scale NH3 mitigation in progress |
Future Plans
New experiments in laboratory and field are needed to further improve NH3 mitigation and capturing efficiencies of the GPM system by modifying concentrations of acidic solution, changing GPM tube dimensions and morphology, and increasing the acid solution circulation flow rate in the GPM tube.
Authors
Saqib Mukhtar, Professor, Biological & Agricultural Engineering Department, Texas A & M University System, mukhtar@tamu.edu
Amir M. Samani Majd, PhD Candidate, Biological & Agricultural Engineering Department, Texas A & M University
Additional Information
1- An Investigation of Ammonia Extraction from Liquid Manure Using a Gas-Permeable Membrane. Available at: http://elibrary.asabe.org/azdez.asp?JID=5&AID=37764&CID=loui2011&T=2
2- Application of Diluted Sulfuric Acid for Manure Ammonia Extraction Using a Gas-Permeable Membrane. Available at: http://elibrary.asabe.org/azdez.asp?JID=5&AID=42102&CID=dall2012&T=2
3- AFO Ammonia Mitigation Technology for Sustainable Environmental Stewardship
Acknowledgements
Funding for this study was provided through a grant by the United States Department of Agriculture: National Institute for Food and Agriculture (UDSA- NIFA).
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2013. Title of presentation. Waste to Worth: Spreading Science and Solutions. Denver, CO. April 1-5, 2013. URL of this page. Accessed on: today’s date.

