This is a self-guided learning lesson about greenhouse gases (GHG) and their connections to livestock and poultry production. It is useful for self-study and for professionals wishing to submit continuing education credits to a certifying organization. Anticipated time: 60 minutes. At the bottom of the page is a quiz that can be submitted and a score of 7 out of 10 or better will earn a certificate of completion. (Teachers/educators: visit the accompanying GHG curriculum materials page)
Module Topics
- Why does climate change?
- How does US agriculture to compare to other industries and worldwide agriculture?
- What greenhouse gases (GHG) are emitted by livestock and poultry farms?
- What are mitigation and adaptation strategies
What is Climate Change?
Download and read “Why Does Climate Change?” (PDF; 8 pages). Includes basics and terminology about natural and man-made drivers of climate change.
US Agriculture Comparisons to Other Industries and Worldwide Agriculture
Watch this short video “Agriculture and Greenhouse Gases: Some Perspective” (5 minutes). This also includes some very good reasons why farmers, ranchers, and ag professionals should care about the topic of climate change, regardless of political stances on solutions.
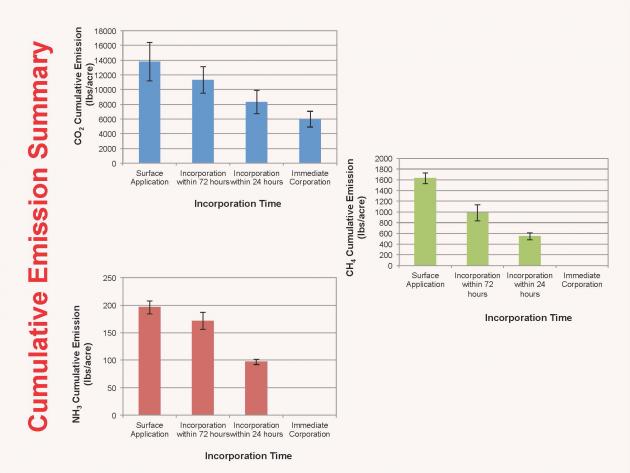
Greenhouse Gases Emitted by Livestock, Poultry. and Other Agricultural Activities
Watch this short video discussing the most important gases produced through livestock, poultry, and cropping activities on farms and ranches. (8 minutes)
Review the following fact sheet:
- Greenhouse Gas Emissions from Livestock and Poultry (you can also create a printer-friendly version)
Mitigation and Adaptation
Watch this short video “Carbon, Climate Change, and Controversy” by Marshall Sheperd, University of Georgia (4 minutes)
Watch this video on “Mitigation and Adaptation: Connections to Agriculture” (13 minutes)
Quiz
When you have completed the above activities, take this quiz. If you score at least 7 of 10 correct, you will receive a certificate of completion via email. If you are a member of an organization that requires continuing education units (CEUs), we recommend that you submit your certificate to them for consideration as a self-study credit (each individual organization usually has a certification board that decides which lessons are acceptable). Go to quiz….
American Registry of Professional Animal Scientist (ARPAS) members can self-report their completion of this module at the ARPAS website.
Acknowledgements
Author: Jill Heemstra, University of Nebraska-Lincoln
Building Environmental Leaders in Animal Agriculture (BELAA) is a collaborative effort of the National Young Farmers Educational Association, University of Nebraska-Lincoln, and Montana State University. It was funded by the USDA National Institute for Food and Agriculture (NIFA) under award #2009-49400-05871. This project would not be possible without the Livestock and Poultry Environmental Learning Community and the National eXtension Initiative.