Table of Contents
 Anaerobic Digestion Nutrient Transformations
Anaerobic Digestion Nutrient Transformations
Anaerobic digestion (AD) is the process in which organic compounds are broken down by naturally occurring bacteria, including methanogenic microorganisms under oxygen free conditions, transforming organic matter into biogas (methane (CH4), carbon dioxide (CO2), water vapor, ammonia, and hydrogen sulfide) (Dugba and Zhang, 1999; AgSTAR, 2010b). The end products of this process include biogas, a renewable energy source, and treated manure containing plant nutrients that can be used to replace agricultural fertilizers. The nitrogen, (N), phosphorus (P), and potassium (K) are not lost or reduced due to the AD process, but are transformed (see figure 1) from organic forms to inorganic forms while the carbon is converted to biogas (Table 1). As a result, the levels of ammonium N and inorganic P increase as a percent of total N and total P when compared to raw manure. The increase in ammonium content will vary due to pre-digester management of the manure. Data from Washington State University indicates that ~ 40% of the N going into the AD is NH4+-N, and 60 % of N coming out of the AD is NH4+-N (O’Rourke et al, 2009 SWCS presentation)

Chemical Composition of Digested Effluent
Nitrogen
Nitrogen that enters a digester from dairy manure is either in the ammonium or organic form. Much of the organic nitrogen is converted via nitrogen mineralization during the digestion process to ammonium, raising the overall level of ammonium in the effluent (Field et al., 1984). Although a small amount of ammonia gas will be lost to biogas, the total nitrogen leaving the digester is generally considered equal to that added to the digester. (Topper, 2006).
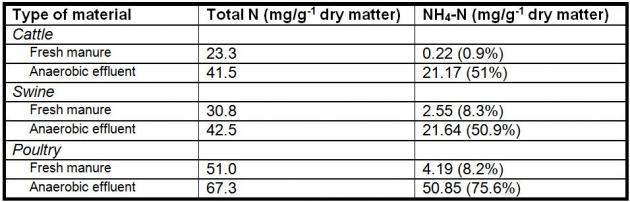
Nutrient content of the AD input will vary depending on the species of the contributing manure and if there is any addition of other organic feedstocks (co-digestion). Kirchmann and Witter (1992; Table 1) evaluated fresh and anaerobically digested manure from three different species for nutrient concentration. They conclude that anaerobic digestion of manure resulted in higher ammonium N concentrations (50-75% of total N) in the digested material. In a similar project with co-digestion of dairy manure and pre-consumer waste feedstocks, they observed an increase from 34% NH4 (% of total N) in pre-AD material to 58% NH4 (% of total N) post-AD material prior to liquid-solid separation (7.3 NH4 and 21 total N lbs./ 1000gal before digestion; 9.1 NH4 and 15.8 total N lbs./1000 gal post digestion, Whitefield, 2009). Anaerobic digestion facilitates nitrogen mineralization, while carbon is converted to biogas. Additionally, carbon is partially removed from the digested material, reducing the C:N ratio (Kirchmann and Witter, 1992; Moller et al., 2008).
Table 1. Forms of nitrogen in fresh manure and anaerobic digestion effluent (Kirchmann et al., 1992; numbers in parenthesis are the percent of total N).
Phosphorus
Nutrient speciation data collected from previous AD studies suggest that a high percentage of the P can be found in the inorganic form in the AD effluent (Wrigley et al., 1992; Bowers et al., 2007; Marti et al., 2008; Moody, 2009). Moody (2009) and colleagues demonstrated a 26% increase of inorganic P (PO43) in digested swine slurry compared to the raw swine slurry (1591 mg/L and 1256.2 of PO43– respectively). Inorganic P is comprised of soluble and insoluble orthophosphates and polyphosphates. When evaluating the nutrient transformation of five different types of ADs in New York, the percent change in orthophosphorus (OP) after digestion varied from 7-27% depending on the type of AD (Figure 2). The case study data from Cornell (Figure 2) also demonstrates the percent change of total Kjeldahl nitrogen (TKN), ammonia nitrogen (NH3-N), organic nitrogen (ON), and TP (Gooch et al, 2006). The positive percent change indicates a greater concentration of the nutrient in the post digested effluent compared to the influent vs. a negative percent change which indicates the nutrient is more concentrated in the influent before digested compared to after digestion. These data represents several farms with digesters, some with different digester models, and therefore variation would be expected. Bowers et al., (2007) demonstrated total phosphorus (TP) content ranging from 238 to 323 ppm, from which OP contributed 106 to 231 ppm of the TP in the post digestion effluent of a co-digestion dairy manure AD. Also one should not expect loss of N or P during digestion, and variability due to sampling and analyses could have caused some error in mass balance calculations.
Figure 2. Nutrient Transformation (% change) of five different farms with digesters from a case study in NY state (Source: Gooch et al, 2006) The percent changes was calculated as influent nutrient value minus effluent nutrient value; therefore even though there is a negative percent change of NH3-N and OP, there is a greater amount in the digested effluent compared to the influent.

pH and Chemical Oxygen Demand
The pH of the manure remains fairly neutral throughout digestion maintaining microbial stability within the digester (Wen, 2009). In a study by Wang and collegues (2010) anaerobic digestion reduced manures’ chemical oxygen demand and amount of total and volatile solids by 30-40%.
Organic nitrogen is mineralized to ammonium while conserving total nitrogen and phosphorus (Wang et al., 2010). The Danish Biogas Institute reports 25% more available NH4-N and higher pH in AD manures (Monnet, 2003).
Liquids-Solids Separation
Many AD systems are managed with the use of liquids-solids separation after the manure has been digested. Separating out the solids for use as a soil amendment or bedding can result in a small reduction of nutrients in the remaining fraction. Preliminary data from Washington State University suggests that 27 % of the solids, 6 % of the N, and 8 % of the P are removed in the solids from AD treated manure (screw type solids separator).
Potential for Increased Efficiencies
Although there is a growing body of research on anaerobic digestion and crop nutrient availability, this technology has not been extensively studied. Most work has been focused on short term nutrient recovery (1-3 years), and not the long term impacts (5+ years) of fertilizing with AD manures (Arthurson, 2009).
AD manure has been shown to have the same positive effects on yield and crop production when applied at equal rates of plant-available N as synthetic fertilizers or raw manures in corn and forage production systems (Morris and Lathwell, 2004; Loria et al., 2007), while soil quality and fertility indicators are improved relative to synthetic fertilizers (de Boer, 2008; Arthurson, 2009).
Since anaerobically digested dairy manure could provide more plant available nitrogen than untreated manure (Kirchmann and Witter, 1992; Michel et al., 2010), the potential exists for increasing agricultural efficiences (Morris and Lathwell, 2004; Moller and Stinner, 2010). Increased concentrations of NH4-N in AD manure would increase the potential for N loss in the field, so best management practices would be required to take advantage of the higher NH4-N content.
Application of AD dairy manure to corn has been shown to produce similar total plant N uptake and equivalent or greater yields than inorganic fertilizer. Early growth/yield of corn was greater from application of AD dairy manure than from synthetic fertilizers on acidic soils (<7.0) but not on alkaline soils (Morris and Lathwell, 2004). The acidic soils restrict ammonia loss from ammonium-rich AD manure resulting in greater N uptake (Nelson, 1982).
Figure 3 summarizes information from 2 years of a study (Saunders, 2011) conducted to look at anaerobically digested dairy manure or undigested dairy manure. When anaerobically digested or undigested manure was applied at equal amounts of total nitrogen, equivalent amounts of dry matter yield (~ 7.5 tons) and similar amounts of nitrogen uptake (~ 475 pounds) were observed. The control did not receive any form of fertilizer or manure, and was stastically different (P<.05) from the manure treatments before and after digestion.
Figure 3. Average annual yield of dry matter and annual nitrogen uptake by grass receiving equal amounts of nitrogen from anaerobically digested dairy manure or undigested dairy manure.

Manure serves as a useful, low-cost source of nutrients for crop production (Sommerfeldt et al., 1988; Jokela, 1992; Ferguson et al., 2005; Nyiraneza and Snapp, 2007). Anaerobically digested manure provides sufficient nutrients to support biomass and crop yields equivalent to synthetic fertilizers and raw manures (Bittman et al., 1999; Loria et al., 2007). Some studies, (Rubaek, 1996; Chantigny et al., 2007; de Boer, 2008) have found increased yield and nitrogen availability with application of anaerobically digested material as compared to non-digested material, possibly due to increased nitrogen content and reduced carbon content, which can result in nitrogen mineralization by microbes. In addition, manure applications to soils have enhanced soil quality and fertility compared to soils receiving synthetic fertilizers (de Boer, 2008; Arthurson, 2009). A crop will typically recover <50% of applied fertilizer nitrogen (Stevens et al., 2005). Up to 46% of applied manure nitrogen may be left over in the soil at the end of the growing season, increasing the potential for loss, after multiple applications during a season (Munoz et al., 2003). Over-application of manure nitrogen in excess of crop uptake can result in nitrate leaching (Angle et al., 1993). Some studies have indicated that manure nitrogen poses an equal or slightly less risk to leaching than synthetic fertilizers (Jokela, 1992;Trindade et al., 2009). Others have determined manure increases nitrate leaching (Jemison and Fox, 1994). During winter months when plants are dormant, nitrate leaching can be the main source of N loss (Bakhsh et al., 2007). The shift in organic to inorganic nutrients during the AD process should be considered when developing a farm nutrient management plan.
References
-
- AgSTAR Program. 2002. Managing manure with biogas recovery systems improved performance at competitive costs. Environmental Protection Agency, Office of Air and Radiation. 430-F02-004 http://www.epa.gov/agstar/documents/manage.pdf.
- Amon, B., V. Kryvoruchko, T. Amon, and S. Zechmeister-Boltenstern. 2006. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agriculture Ecosystems & Environment 112:153-162.
- Angle, J.S., C.M. Gross, R.L. Hill, and M.S. McIntosh. 1993. Soil nitrate concentrations under corn as affected by tillage, manure, and fertilizer applications. Journal of Environmental Quality 22:141-147.
- Arthurson, V. 2009. Closing the global energy and nutrient cycles through application of biogas residue to agricultural land – potential benefits and drawbacks. Energies 2:226-242.
- Bakhsh, A., R.S. Kanwar, C. Pederson, and T.B. Bailey. 2007. N-source effects on temporal distribution of NO3-N leaching losses to subsurface drainage water. Water Air and Soil Pollution 181:35-50.
- Brady, N.C., and R.R. Weil. 2002. The Nature and Property of Soils. 13 ed. Prentice Hall, Upper Saddle River, New Jersey.
- Bittman, S., C.G. Kowalenko, D.E. Hunt, O. Schmidt. 1999. Surface-banded and broadcast dairy manure effects on tall fescue yield and nitrogen uptake. Agronomy Journal 91: 826-833.
- Bowers, K.E., T.X. Zhang and J.H. Harrison. 2007. Phosphorus removal by struvite crystallization in various livestock wastewaters. American Society of Agricultural and Biological Engineers International Air and Waste Symposium (September 16-19, Broomfield, Colorado). Publication 701P0907cd of the ASABE. St Joseph, MI.
- Chantigny, M.H., D.A. Angers, P. Rochette, G. Belanger, D. Masse, and D. Cote. 2007. Gaseous nitrogen emissions and forage nitrogen uptake on soils fertilized with raw and treated swine manure. Journal of Environmental Quality 36:1864-1872.
- de Boer, H.C. 2008. Co-digestion of animal slurry can increase short-term nitrogen recovery by crops. Journal of Environmental Quality 37:1968-1973.
- Dugba, P.N., and R.H. Zhang. 1999. Treatment of dairy wastewater with two-stage anaerobic sequencing batch reactor systems – thermophilic versus mesophilic operations. Bioresource Technology 68:225-233.
- Field, J.A., J.S. Caldwell, S. Jeyanayagam, R.B. Reneau, W. Kroontje, and E.R. Collins. 1984. Fertilizer recovery from anaerobic digesters. Transactions of the American Society of Agricultural and Biological Engineers 27:1871-1876.
- Ferguson, R.B., J.A. Nienaber, R.A. Eigenberg, and B.L. Woodbury. 2005. Long-term effects of sustained beef feedlot manure application on soil nutrients, corn silage yield, and nutrient uptake. Journal of Environmental Quality 34:1672-1681.
- Fox, R.H., W.P. Piekielek, and K.E. Macneal. 1996. Estimating ammonia volatilization losses from urea fertilizers using a simplified micrometeorological sampler. Soil Science Society of America Journal 60:596-601.
- Gale, E.S., D.M. Sullivan, C.G. Cogger, A.I. Bary, D.D. Hemphill, and E.A. Myhre. 2006. Estimating plant-available nitrogen release from manures, composts, and specialty products. Journal of Environmental Quality 35:2321-2332.
- Gooch, C.A, S.F. Inglis, and P.E. Wright. 2006. Biogas Distributed Generation Systems Evaluation and Technology Transfer – Interim Report. NYSERDA Project No. 6597. New York State Energy Research and Development Authority.
- Jemison, J.M., and R.H. Fox. 1994. Nitrate leaching from nitrogen- fertilized and manured corn measured with zero-tension pan lysimeters. Journal of Environmental Quality 23:337–343.
- Jokela, W.E. 1992. Nitrogen-fertilizer and dairy manure effects on corn yield and soil nitrate. Soil Science Society of America Journal 56:148-154.
- Kirchmann, H., and E. Witter. 1992. Composition of fresh, aerobic and anaerobic farm animal dungs. Bioresource Technology 40:137-142.
- Loria, E.R., J.E. Sawyer, D.W. Barker, J.P. Lundvall, and J.C. Lorimor. 2007. Use of anaerobically digested swine manure as a nitrogen source in corn production. Agronomy Journal. 99: 1119-1129.
- Marti, N., A. Bouzas, A. Seco, and J. Ferrer. 2008. Struvite precipitation assessment in anaerobic digestion processes. Chemical Engineering J. 141: 67‐74.
- Michel, J., A. Weiske, and K. Moller. 2010. The effect of biogas digestion on the environmental impact and energy balances in organic cropping systems using the life-cycle assessment methodology. Renewable Agriculture and Food Systems 25:204-218.
- Moller, K., and W. Stinner. 2010. Effects of organic wastes digestion for biogas production on mineral nutrient availability of biogas effluents. Nutrient Cycling in Agroecosystems 87:395-413.
- Moller, K., W. Stinner, A. Deuker, and G. Leithold. 2008. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutrient Cycling in Agroecosystems 82:209-232.
- Monnet, F. 2003. Digested biomass as fertiliser. Available at: http://www.landbrugsraadet.dk/view.asp?ID=2281(Verified 21 Sept. 2010). Danish Biogas Association.
- Morris, D.R., and D.J. Lathwell. 2004. Anaerobically digested dairy manure as fertilizer for maize in acid and alkaline soils. Communications in Soil Science and Plant Analysis 35:1757-1771.
- Munoz, G.R., J.M. Powell, and K.A. Kelling. 2003. Nitrogen budget and soil N dynamics after multiple applications of unlabeled or (15)Nitrogen-enriched dairy manure. Soil Science Society of America Journal 67:817-825.
- Moody, L., R. Burns, and K.J. Stalder. 2009. Effect of anaerobic digestion on manure characteristics for phosphorus precipitation from swine waste. Appl. Eng. Agric. 25:97-102.
- Nelson, D.W. 1982. Gaseous losses of nitrogen other than through denitrification. In Stevenson, F.J., Bremner, J.M., Hauck, R.D., Keeney, D.R., editors. Nitrogen in Agricultural Soils. Agronomy monograph. Madison, WI: American Society of Agronomy No. 22, 327–363.
- Nyiraneza, J., and S. Snapp. 2007. Integrated management nitrogen and efficiency of inorganic and organic in potato systems. Soil Science Society of America Journal 71:1508-1515.
- Robertson G.P. 2000. Denitrification. Pagesc-181-190 in M.E. Sumner ed. Handbook of Soil Science. CRC Press, Boca Raton, Florida, USA.
- Rubaek, G.H., K. Henriksen, J. Petersen, B. Rasmussen, and S.G. Sommer. 1996. Effects of application technique and anaerobic digestion on gaseous nitrogen loss from animal slurry applied to ryegrass (Lolium perenne). Journal of Agricultural Science 126:481-492.
- Saunders, O. 2011. Environmental benefits and consequences of field applied anaerobically digested dairy manure for forage production. MS Thesis, Master of Science in Soil. Washington State University. Department of Crop and Soil Sciences.
- Sommerfeldt, T.G., C. Chang, and T. Entz. 1988. Long-term annual manure applications increase soil organic-matter and nitrogen, and decrease carbon to nitrogen ratio. Soil Science Society of America Journal 52:1668-1672.
- Stevens, W.B., R.G. Hoeft , and R.L. Mulvaney. 2005. Fate of nitrogen-15 in a long-term nitrogen rate study: II. Nitrogen uptake efficiency. Agronomy Journal 97:1046–1053.
- Topper, P.A., R.E. Graves, and T. Richard. 2006. The fate of nutrients and pathogens during anaerobic digestion of dairy manure. Pennsylvania State, Department of Agricultural and Biological Engineering. G71.
- Trindade, H., J. Coutinho, S. Jarvis, and N. Moreira. 2009. Effects of different rates and timing of application of nitrogen as slurry and mineral fertilizer on yield of herbage and nitrate-leaching potential of a maize/Italian ryegrass cropping system in north-west Portugal. Grass and Forage Science 64:2-11.
- Wang, L., Y.C. Li, P. Chen. M. Min, Y.F. Chen, J.Zhu, and R.R. Ruan. 2010. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresource Technology 101:2623-2628.
- Wen, Z., and S. Chen. 2009. Development of a sequential continuous stirred tank reactor (CSTR) system for anaerobic digestion of liquid dairy manure. American Society of Agricultural and Biological Engineers. Paper number 067070. https://elibrary.asabe.org/abstract.asp?search=1&JID=5&AID=21032&CID=por2006&v=&i=&T=1&urlRedirect=[anywhere=&keyword=&abstract=&title=&author=&references=&docnumber=on&journals=All&searchstring=21032&pg=&allwords=&exactphrase=21032&OneWord=&Action=Go&Post=Y&qu=]&redirType=newresults.asp
- Whitefield, E.M, J.H. Harrison, A. Bary, C. Cogger and A.M. Fortuna. 2009. Nutrient and pathogen characterization in a community anaerobic digester; presentation. Conservation Innovation Grant showcase at the Soil and Water Conservation Society Annual Meeting. July 14, 2009. Dearborn, MI.
- Wrigly,T., K. Webb, and H. Venkitachalm. 1992. A laboratory study of struvite precipitation after anaerobic digestion of piggery wastes. Bioresource Rechnology 41(2): 117-121.
Contributors to this Article
Authors
-
- Joe H. Harrison, Professor, Nutrient Management Specialist, PAS, Washington State University
- Elizabeth Whitefield, Research Associate, PAS, Washington State University
- Olivia Saunders, Graduate Student, graduated May 2011, Washington State University
- Andy Bary, Senior Scientific Assistant, Department of Crop and Soil Sciences, Washington State University Puyallup
Peer Reviewers
- Bill Jokela, Research Soil Scientist, USDA-Agricultural Research Service, Dairy Forage Research Center, Marshfield, WI
- John Classen, Associate Professor Biological and Agricultural Engineering Waste Management, North Carolina State University
Page Manager
- Sandy Anderson, Washington State University