Introduction
Biogas generated from anaerobic digestion processes is a clean and environmentally friendly renewable fuel. But it is important to clean, or upgrade, biogas before using it to increase its heating value and to make it useable in some gas appliances such as engines and boilers.
Biogas Utilization
While most large farms use their biogas for heat and power, it is worthwhile to consider all the options before deciding which path to take, including direct sale of biogas to an off-farm buyer.
Raw animal manure biogas contains 55 to 65% methane (CH4), 30 to 45% carbon dioxide (CO2), traces of hydrogen sulfide (H2S) and hydrogen (H2), and fractions of water vapor. For the anaerobic digestion of sludge or landfill processes, traces of siloxanes may also be found in biogas. These siloxanes mainly originate from silicon-containing compounds widely used in various industrial material or frequently added to consumer products such as detergents and personal care products. This article will not address the cleanup of biogas of siloxanes.
Biogas is about 20% lighter than air and has an ignition temperature in the range of 650 to 750 degrees C. (1,200-1,380 degrees F.). It is an odorless and colorless gas that burns with a clear blue flame similar to that of natural gas. However, biogas has a calorific value of 20-26 MJ/m3 (537-700 Btu/ft3) compared to commercial quality natural gas’ caloric value of 39 MJ/m3 (1,028 Btu/ft3).
Biogas can potentially be used in many types of equipment, including:
- Internal Combustion (Piston) Engine – Electrical Power Generation, Shaft Power
- Gas Turbine Engine (Large) – Electrical Power Generation, Shaft Power
- Microturbine Engine (Small) – Electrical Power Generation
- Stirling Heat Engine – Electrical Power Generation
- Boiler (Steam) Systems
- Hot Water Systems
- Process Heaters (Furnaces)
- Space or Air Heaters
- Gas Fired Chiller – Refrigeration
- Absorption Chiller – Refrigeration
- Combined Heat and Power (CHP) – Large and Small Scale – Electrical Power and Heat
- Fuel Cells – Electrical Power, Some Heat
There are a variety of end uses for biogas. Except for the simplest thermal uses such as odor flaring or some types of heating, biogas needs to be cleaned or processed prior to use. With appropriate cleaning or upgrade, biogas can be used in all applications that were developed for natural gas.
The three basic end uses for biogas are:
- production of heat and steam
- electricity generation
- vehicle fuel
Production of heat or steam
The most straightforward use of biogas is for thermal (heat) energy. In areas where fuels are scarce, small biogas systems can provide the heat energy for basic cooking and water heating. Gas lighting systems can also use biogas for illumination.
Conventional gas burners are easily adjusted for biogas by simply changing the air-to-gas ratio. The demand for biogas quality in gas burners is low, only requiring a gas pressure of 8 to 25 mbar and maintaining H2S levels to below 100 ppm to achieve a dew point of 150 degrees C.
Electricity Generation or Combined Heat and Power (CHP)
Combined heat and power systems use both the power producing ability of a fuel and the inevitable waste heat. Some CHP systems produce primarily heat, and electrical power is secondary (bottoming cycle). Other CHP systems produce primarily electrical power and the waste heat is used to heat process water (topping cycle). In either case, the overall (combined) efficiency of the power and heat produced and used gives a much higher efficiency than using the fuel (biogas) to produce only power or heat.
Other than high initial investments, gas turbines (micro-turbines, 25-100 kW; large turbines, >100 kW) with comparable efficiencies to spark-ignition engines and low maintenance can be used for production of both heat and power. However, internal combustion engines are most cmmonly used in CHP applications. The use of biogas in these systems requires removal of both H2S (to below 100 ppm) and water vapor.
Fuel cells are considered the small-scale power plants of the future for production of power and heat with efficiencies exceeding 60% and low emissions. One of the largest digester/fuel cell units is located in Washington State. The fuel cell, located at the South Treatment Plant in Renton, WA, can consume about 154,000 ft3 of biogas a day to produce up to 1 megawatt (1,000,000 watts) of electricity. That’s enough to power 1,000 households, but it’s being used instead for the operation of the plant.
Vehicle fuel
Gasoline vehicles can use biogas as a fuel provided the biogas is upgraded to natural gas quality in vehicles that have been adjusted to using natural gas. Most vehicles in this category have been retro-fitted with a gas tank and a gas supply system in addition to the normal petrol fuel system. However, dedicated vehicles (using only biogas) are more efficient than these retro-fits.
Biogas Cleanup Or Upgrading
Biogas cleaning is important for two reasons: (1) to increase the heating value of biogas, and (2) to meet requirements for some gas appliances (engines, boilers, fuel cells, vehicles, etc). Desired biogas cleaning or upgrading purposes are summarized in Figure 1. “Full treatment” implies that biogas is cleaned of CO2, water vapor, and other trace gases, while “reforming” is conversion of methane to hydrogen.
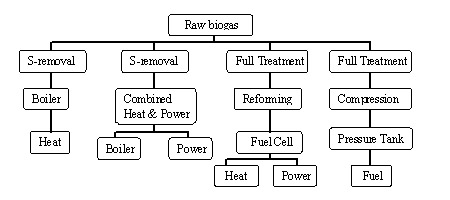
Figure 1. Alternative biogas utilization and required cleanup
CO2 Removal
For many of the simpler biogas applications such as heaters or internal combustion engines or generator systems, carbon dioxide (CO2) removal from biogas is not necessary and CO2 simply passes through the burner or engine. For more demanding biogas/engine applications, such as vehicles that require higher energy density fuels, CO2 is routinely removed. Removing CO2 increases the heating value and leads to a consistent gas quality similar to the natural gas. Carbon dioxide can be removed from biogas economically through absorption or adsorption. Membrane and cryogenic separations are other possible processes.
Pressurized counter-current scrubbing of CO2 and H2S from biogas can be accomplished in water. For removal of CO2 in particular; pH, pressure, and temperatures are critical. High pressures, low temperature, and high pH increases CO2 scrubbing from biogas. Use of Ca(OH)2 solutions can completely remove both CO2 and H2S. Both CO2 and H2S are more soluble in some organic solvents such as polyethyleneglycol and alkanol amines that do not dissolve methane. These organic solvents can thus be used to scrub these gases from biogas even at low pressures. Systems using these kinds of organic solvents can remove CO2 down to 0.5% from the biogas.
However, use of organic solvents is much more expensive than water systems. Adsorption of CO2 on solids such as activated carbon or molecular sieves is possible although it requires high temperatures and pressures. These processes may not be cost-effective because of associated high temperature and pressure drops. Cryogenic separation is possible because at 1 atm, methane has a boiling point of -106oC, whereas CO2 has a boiling point of -78oC. Fractional condensation and distillation at low temperatures can thus separate pure methane in liquid form, which is convenient for transportation. Up to 97% pure methane can be obtained, but the process requires high initial and operational investments. Membrane or molecular sieves depend on the differences in permeability of individual gas components through a thin membrane. Membrane separations are quickly gaining in popularity. Other chemical conversions are technically viable, but their economics are poor for practical biogas-cleaning.
Water Vapor Removal
Straight from the digester, biogas will generally be saturated with vapor. Besides reducing the energy value of biogas, water can react with H2S to create ionic hydrogen and/or sulfuric acid, which is corrosive to metals. Refrigeration or sensible pipe-work design can condense and remove the water. The biogas is normally compressed before cooling to achieve high dew points. Alternative water vapor removal mechanisms include adsorption on: (1) silica gel and Al2O3 at low dew points, (2) glycol and hygroscopic salts at elevated temperatures, and 3) molecular sieves.
Removal of Hydrogen Sulfide
Hydrogen sulfide in biogas needs to be removed for all but the most simple burner applications. Hydrogen sulfide in combination with the water vapor in raw biogas can form sulfuric acid (H2SO4), which is very corrosive to engines and components. At concentrations above 100 parts per million by volume (ppmv), H2S is also very toxic. Activated carbon can be used to remove both H2S and CO2. Activated carbon catalytically converts H2S to elemental sulfur. Hydrogen sulfide can also be scrubbed out from biogas in either: NaOH, water, or iron salt solutions. A simple and inexpensive process is dosing a stream of biogas with O2, which oxidizes H2S to elemental sulfur. Oxygen dosing can reduce H2S to below 50ppm levels from biogas [Warning: IMPROPERLY DOSING A BIOGAS STREAM WITH O2 CAN CREATE AN EXPLOSION HAZARD]. Iron oxide also removes H2S as iron sulfide. This method can be sensitive to high water vapor content of the biogas. In addition to clean up of biogas of H2S after it has been produced, available methods of reducing H2S content from produced biogas include: co-digestions, multiphase digestion, reactor pH buffering, and removal of sulfur from feed substrates.
Conclusions
Biogas produced from animal waste can be a valuable energy resource. By combusting waste methane (biogas), a powerful greenhouse gas is eliminated that would otherwise be released. If used in simple burners for cooking or lighting the gas may not need to be treated prior to use. However, for uses that require the gas to be used in internal combustion engines, boilers or fuel cells, the biogas will probably need to be pretreated in order to remove corrosive or dangerous contaminants. The primary contaminant of biogas is hydrogen sulfide. This chemical will also react with water to form corrosive acids that can attack metals and plastics. Hydrogen sulfide is also toxic and sufficient quantities also present a possible health hazard if not treated.
Additional Resources
Intro | Feedstocks | Processing | Utilization
References
- Appels, L., J. Baeyens, J. Degre`ve, R. Dewil. 2008. Principles and potential of the anaerobic digestion of waste-activated sludge. Progress in Energy and Combustion Science, 34:755–781.
- Drewitz, M., P.Goodrich. 2005. Minnesota dairy runs hydrogen fuel cell on biogas. https://www.biocycle.net/minnesota-dairy-runs-hydrogen-fuel-cell-on-biogas/
- EMG International, Inc. 2007. Biogas Clean-Up Technologies. Presentation to NYS ERDA Innovations in Agriculture.
- FAO. 1997. A system approach to biogas technology, http://www.fao.org/3/ae897e/ae897e00.htm
- Glub, J.C., L.F. Diaz. 1991. Biogas purification process. Biogas and alcohol fuels production, vol. II. The JP Press.
- Hagen, M., E. Polman. 2001. Adding gas from biomass to the gas grid. Final report submitted to Danish Gas Agency, pp. 26-47.
- Harasmowicz, M., P. Orluk, G. zakrzewska-Trznadel, A.G. Chemielewski. 2007. Application of polyimide membranes for biogas purification and enrichment. Journal of Hazardous Materials 144:698-702.
- Kapdi, S.S., V.K. Vijay, S.K. Rajesh, R. Prasad. 2005. Biogas scrubbing, compression and storage: perspective and prospectus in Indian context. Renewable Energy 30:1195-1202.
- Kayhanian, D.J. Hills. 1988. Membrane purification of anaerobic digester gas. Biological Wastes 23: 1-15.
- Lastella, G., C. Testa, G. Cornacchia, M. Notornicola, F. Voltasio, V.K. Sharma. 2002. Anaerobic digestion of semi-solid organic waste: biogas production and its purification. Energy Convers. Manage., 43:63–75.
- Leposky, G. 2005. Fuel Cell Uses Biogas from Sewage to Generate Electricity. https://www.distributedenergy.com/home/article/13003393/fuel-cell-uses-biogas-from-sewage-to-generate-electricity
- Li, K., W.K. Teo. 1993. Use of an internally staged permeator in the enrichment of methane from biogas. J. Membr Sci 1993;78:181–90.
- Martin, J. H. 2008. A method to Evaluate Hydrogen Sulfide Removal from Biogas. MS Thesis: Biological and Agricultural Engineering, North Carolina State University, Raleigh, North Carolina.
- Pandey, D.R., C. Fabian. 1989. Feasibility studies on the use of naturally accruing molecular sieves for methane enrichment from biogas. Gas Separation and Purification, 3:143-147.
- Sarkar, S.C., A. Bose. 1997. Role of activated carbon pellets in carbon dioxide removal. Energy Conversions Management 38:S105-S110.
- Stern, S.A., B. Krishnakumar, S.G. Charati, W.S. Amato, A.A. Friedmann, D.J. Fuess. 1998. Performance of a bench-scale membrane pilot plant for the upgrading of biogas in a wastewater treatment plant. J. Membr Sci, 151:63–74.
- Walls, J.L., C. Ross, M.S. Smith, S.R. Harper. 1989. Utilization of biogas. Biomass 20: 277-290.
- Wellinger, A., A. Lindberg. 2005. Biogas Upgrading and Utilization. Task 24: Energy From Biological Conversion of Organic Waste. IEA Bioenergy. http://www.biogasmax.eu/media/biogas_upgrading_and_utilisation__018031200_1011_24042007.pdf
- Wise, D.L. 1981. Analysis of systems for purification of fuel gas. Fuel gas production from biomas, vol. 2. Boca Raton, FL. The CRC Press.
Contributors
Authors
Peer Reviewers
![]() Waste to Worth home | More proceedings….
Waste to Worth home | More proceedings….


 Harnessing energy from livestock waste.
Harnessing energy from livestock waste.

