Purpose
Growers often use total nitrogen (N) concentration of dairy manure to estimate plant available N for crop production. This estimate often does not take into account the role soil properties may have on N mineralization (Nmin) rates. This study aims to determine how soil properties impact Nmin rates of dairy manure and composted dairy manure by aerobic incubation. The soil properties investigated, including soil texture, percent organic matter, pH, EC, buffer pH, NO3-N, NH4-N, Olsen P, K, Ca, Mg, Na, CEC, S, Zn, Fe, Mn, Cu, B, and CaCO3 equivalent, which are all accessible to producers sending soil samples to a commercial soil laboratory. The goal of this project is to incorporate soil properties into N availability prediction models for dairy manure to improve N use efficiency of field-applied manure.
What Did We Do?
A total of 16 different soil series were sampled throughout Oregon, Washington, and Idaho in major dairy producing counties at a 12-inch depth. These soils represent over 1.6 million acres in the Pacific Northwest (PNW). One solid dairy manure was sampled in Idaho and one composted dairy manure was sampled in Oregon to be applied to the soils during incubation. All the soils were analyzed for a full suite of soil physiochemical properties at a local soil testing laboratory. The manures similarly received a full analysis at the same laboratory.
We conducted a 12-week incubation of manure-amended soils at 77°F (25°C), sampling periodically for nitrate and ammonium to determine the difference in Nmin rates with changes in soil physiochemical properties. Approximately 1.1 lbs (500 g) of soil was added to 1-gallon Ziplock bags and brought to 80% field capacity. The soils were treated with dairy manure, composted manure, or no manure at a rate of approximately 400 lb N/acre (200 mg N/kg soil) with four replicates for each soil and treatment. Each of the 192 samples were randomly assigned a sample number corresponding to their location inside the incubator. The closed and loosely rolled bags were stored in 12 by 9 by 7-inch cardboard boxes, then placed inside an incubator at 77°F for 12 weeks. Soils were sampled at weeks 0, 2, 4, 8, and 12, where part of the sample was used to monitor soil moisture, and the other was frozen for future analysis. Analysis of the frozen samples for nitrate and ammonium content was conducted using a microplate spectrophotometer using vanadium (III) chloride and sodium salicylate methods, respectively.
What Have We Learned?
The analysis of frozen samples has just begun at the time of submission. Initial results will be available on the poster presented.
Future Plans
The next steps of this project are to conclude the nitrate and ammonium analysis of the soil samples and create Nmin curves with this data for each soil and treatment. These curves will be analyzed to determine if the differences in Nmin rates correlate with any of the tested soil physiochemical properties and which properties are most influential. Finally, we will create a model based on correlation data to express the changes in nitrogen mineralization depending on soil physiochemical properties that can be used by producers to adjust their dairy manure application rates depending on their soil test results.
Authors
Presenting author
Ryan A. Auld, Soil Science Graduate Student, Oregon State University
Corresponding author
Amber Moore, Extension Soil Fertility Specialist, Oregon State University, Amber.moore@oregonstate.edu
Additional authors
Jennifer Moore, Research Soil Scientist, Forage Seed and Cereal Research Unit, U.S. Department of Agriculture Agricultural Research Service; Yakun Zhang, Associate Professor, Oregon State University; Christopher Rogers, Research Soil Scientist, Northwest Irrigation and Soils Research, U.S. Department of Agriculture Agricultural Research Service
Additional Information
Acknowledgements
I’d like to acknowledge the BUILD Dairy program and the Oregon Dairy Farmers Association for their support of this project, as well as the many producers who have allowed me to sample soils from their farms.
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2025. Title of presentation. Waste to Worth. Boise, ID. April 7–11, 2025. URL of this page. Accessed on: today’s date.


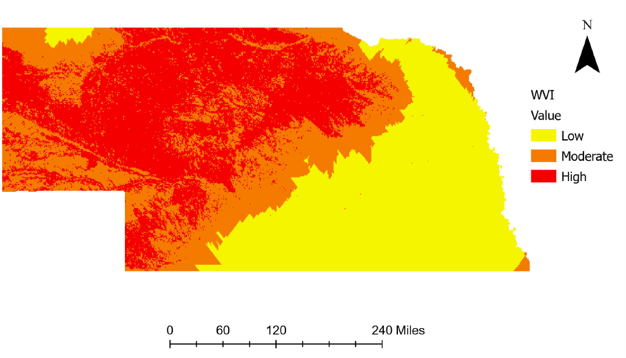







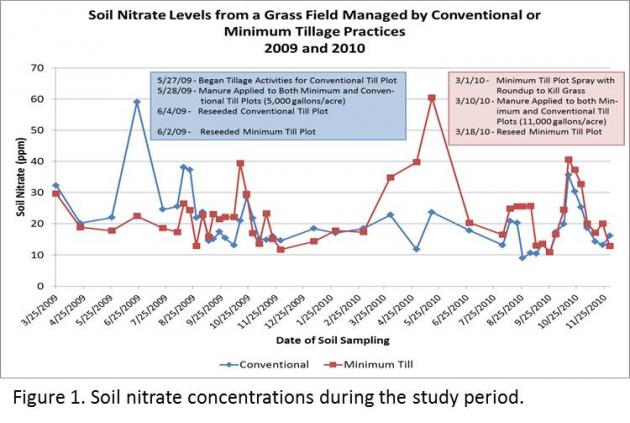
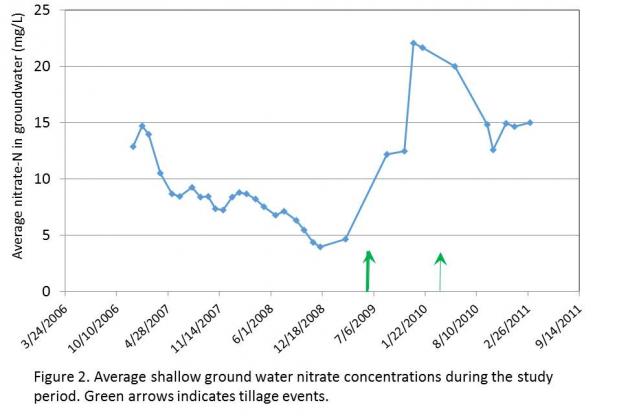
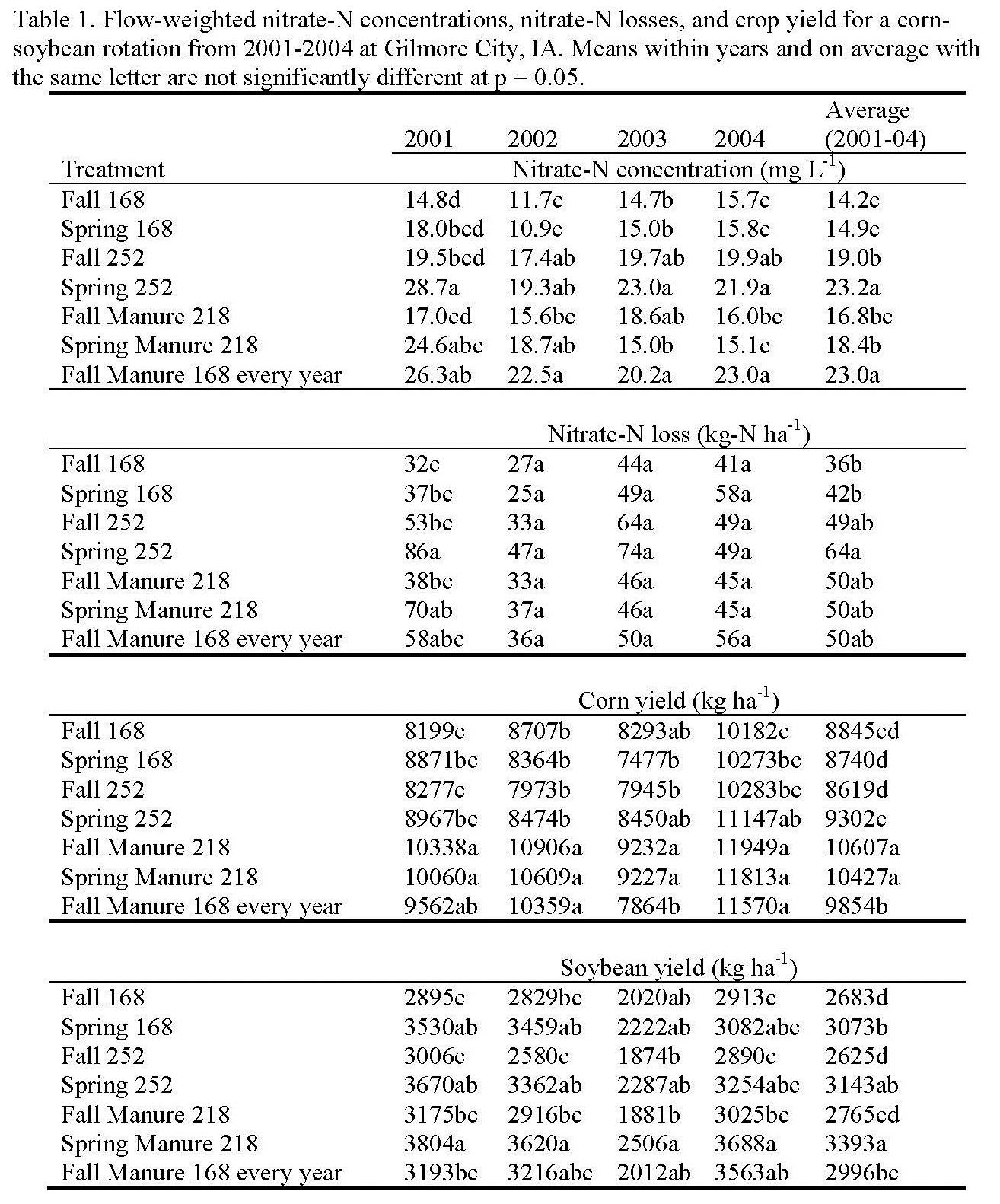
 ased engagement from our dairy producers, with many checking the results map regularly for updates. The map is symbolized with graduated stop light symbology, with poor water quality shown in red and good in green. If they see a red dot or “hot spot” in their neighborhood they may stop us on the street, send an email, or call with ideas or observations of what they believe may have influenced water quality. It has opened the door to conversations and partnerships in identifying and correcting possible influences from their farm.
ased engagement from our dairy producers, with many checking the results map regularly for updates. The map is symbolized with graduated stop light symbology, with poor water quality shown in red and good in green. If they see a red dot or “hot spot” in their neighborhood they may stop us on the street, send an email, or call with ideas or observations of what they believe may have influenced water quality. It has opened the door to conversations and partnerships in identifying and correcting possible influences from their farm. We conducted a scientific peer review of EPA’s project design, methodologies, compliance with EPA’s own Quality Assurance Project Plan/Quality Control procedures, evaluated the overall study design/site selection as described in the three (3) reports, addressed specific data collection concerns and analyzed the results and conclusions drawn from the data as described in the main body of the Report to determine if EPA’s methodology and conclusions were supported by data. We analyzed the overall study design, hydrogeological characterizations, well completions, sample collection (green water, soils and ground water), geochemistry, stable isotopic and organic compound analyses and EPA’s conclusions from these efforts.
We conducted a scientific peer review of EPA’s project design, methodologies, compliance with EPA’s own Quality Assurance Project Plan/Quality Control procedures, evaluated the overall study design/site selection as described in the three (3) reports, addressed specific data collection concerns and analyzed the results and conclusions drawn from the data as described in the main body of the Report to determine if EPA’s methodology and conclusions were supported by data. We analyzed the overall study design, hydrogeological characterizations, well completions, sample collection (green water, soils and ground water), geochemistry, stable isotopic and organic compound analyses and EPA’s conclusions from these efforts.