 Vaccination/PRRSv | Antibiotics and Alternatives | Salmonella
Vaccination/PRRSv | Antibiotics and Alternatives | Salmonella
Why Study Health of Pigs In Relation to Greenhouse Gas (GHG) Emissions?
 Our working hypothesis is that immune activation, from clinical and subclinical disease, reduces growth performance and concomitantly increases nutrient excretion and subsequent GHG emissions from manure management.
Our working hypothesis is that immune activation, from clinical and subclinical disease, reduces growth performance and concomitantly increases nutrient excretion and subsequent GHG emissions from manure management.
Findings from this project will provide validated information for incorporation into the animal physiology models.
Project Objectives
Vaccination/PRRSV Trials
- Evaluate impact of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) exposure and vaccination on animal performance, manure output and composition, and greenhouse gas (GHG) emissions from stored manure.
Antibiotics and Antibiotic Alternatives
- Evaluate the effects of health status on GHG emissions and carbon and water footprints
Salmonella
- Evaluate effects of health status (Salmonella) on animal performance, manure output and composition, and GHG emissions from stored manure.
Research Summary: What Have We Done? What Have We Learned?

Manure reactors installed at Virginia Tech for the pilot study. The manifold on the wall distributes a constant stream of air to each bioreactor, and forces gas through the exhaust lines into the gas analyzers |

Thermo Scientific analyzers for carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), mono-nitrogen oxides (NOx), ammonium (NH4), and hydrogen sulfide (H2S). A programmable manifold switch will sample gas from each reactor sequentially throughout the day. |
Vaccination/PRRSV
Experimental Design:
Pigs were randomly assigned to a 2 x 2 factorial design investigating the interaction of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) vaccination (with or without) and exposure to the PRRSV. One week after weaning, pigs were treated intramuscularly with 2.0 mL of a commercial PRRSV vaccine (Ingelvac PRRS MLV) or sterile saline and housed 4/pen (2 barrows and 2 gilts). Pigs were inoculated 3 weeks after vaccination with sterile medium or PRRSV (MN184). This vaccination protocol has provided high protection against experimental PRRSV infection. (Thacker et al., 2000). Blood samples were collected from pigs upon arrival to the biosafety laboratory to corroborate PRRSV-naïve status. Pigs were given antibiotic-free diets that meet or exceed all nutrient recommendations (NRC, 1998). Growth performance, manure output and composition, and GHG emissions were determined from manure collected from each pen. The study was carried-out for 4 weeks after PRRSV inoculation and used a total of 3 experimental units (i.e., pens) per treatment (i.e., 48 pigs in total). The mitigation strategy tested was the effect of vaccination on pig growth and GHG emissions.
Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) infection caused significant reductions in feed intake which led to reductions in rates of gain and body weight. The infection also caused a reduction in diet digestibility leading to greater manure nutrient output per unit of feed intake and increased greenhouse gas (GHG) production from the stored manure. The impact on GHG production is particularly striking when the data are expressed as litters of gas per kilogram of body weight gain. The increased gas production combined with reduced rates of gain results in more than a tripling of gas production per unit of gain for all of the gases. Vaccination against PRRSV appeared to offer little benefit in terms of animal performance, manure nutrient output, or gas production from manure. Results of the gas production data are presented in Table 1.

Antibiotics and Antibiotic Alternatives
Experimental Design: Seven hundred twenty-four, mixed sex pigs were placed in 11 rooms at the SERB to determine the effects of rearing pigs without antibiotics on growth performance. Pigs were blocked by (body weight) BW and gender and allotted to room and pen with 10/11 mixed-sex pigs/pen. Control pigs consumed diets (Table 2) containing antibiotics and were treated with injectable antibiotics when deemed necessary. Antibiotic-free animals consumed diets with alternatives to antibiotics and received no injectable antibiotics. If sick animals did not respond to antibiotic alternatives, they were removed from the experiment.
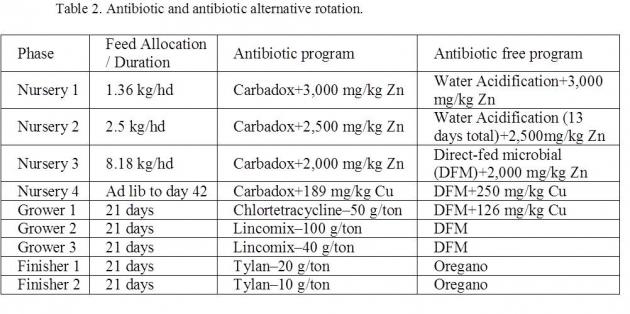
Pigs were weighed at the start and end of each dietary phase, and mortality and morbidity were recorded daily. Data were analyzed using the general linear model (GLM) procedure in SAS (statistical software). During the nursery phase, control pigs grew faster (P<0.02; 0.449 vs 0.426 kg/d), and consumed more feed (P<0.05; 0.694 vs. 0.660 kg/d) than antibiotic free animals, resulting in similar (gain to feed ratio) G:F.
Similar average daily gain (ADG), average daily feed intake (ADFI) and G:F were observed throughout the grower phases, and therefore the increased BW of control-fed pigs was maintained and tended (P=0.06) to be heavier at the start of the finisher phases (86.0 vs. 84.5 kg). However, antibiotic-free animals grew 3% faster (P<0.01) and had 6% better G:F (P<0.001) in the finisher phases.
As a result, there was no overall effect (P>0.10) of treatment on ADG, but there was a trend (P = 0.08) for increased ADFI (2.11 vs. 2.07 kg) and reduced (P<0.05) G:F (0.518 vs. 0.527) in control pigs compared to antibiotic-free. Thirty antibiotic-free animals (8.3%) were removed from the study compared to 11 control (3.0%). In conclusion, antibiotic-free management can yield a similar growth performance to conventional systems, but the limited disease treatment options may limit the number of pigs marketed under this management system.
Salmonella Trial
Experimental Design:
The objective of the study was to determine the impacts of a dirty environment leading to increased pathogen load and salmonella infection on animal performance, manure output and composition, and GHG production from the stored manure. 24 3-week old pigs were transported from the VT swine facility to the Biosafety Laboratory on campus and randomly allotted to one of the following health statuses: 1) High (clean room); 2) Medium (replicated clean on-farm environment); 3) Low (replicated “dirty” farm environment); or 4) Low + Salmonella challenge. Pigs were housed in individual metabolism stalls and fed the same antibiotic free diet as for the VT PRRSV trial. All pigs were assessed for fecal salmonella shedding which is indicative of an active infection upon arrival and found to be negative, and the feed was checked for salmonella contamination and found to be negative. After 10 days of adjustment (31 d of age), pigs allotted to the infected group were orally inoculated with 1×10^9 CFU of Salmonella enterica serotype enterica serovar Typhimurium strain DT104 (ATTC; BAA-185, Manassas, Virginia), and pigs were monitored for growth rate and fecal output and composition for an additional 24 d. Manure was collected each day and the loaded into the manure storage containers. Gas production from the storage containers was assessed continuously throughout the day every other day for the duration of the experiment plus an additional 11 days after the animal trial ended.
Pig inoculated with salmonella exhibited elevated rectal temperatures for 4 d post-innoculation, and shed salmonella in feces for the full 19 days that fecal shedding was monitored. Maintaining pigs in a dirty environment (heavy fecal contamination of the pens) and salmonella infection resulted in equal reductions in the rate of gain and numerical reductions feed efficiency as compared to control animals housed in a clean environment. Emissions of methane from stored manure per unit of weight gain was increased for both the dirty group and the salmonella group by more than 3 fold; and emissions of CO2 and N2O were increased by almost 50% for the dirty group and by 2 fold for the salmonella group.
The effect of Salmonella infection on the gut microbiome are currently being determined. Correlations between greenhouse gas production and key microbial population members will be determined.
The impact of PRRSV and salmonella infections on feed intake was modeled as a time dependent process relative to initial infection and incorporated into the NRC growth model. Although PRRSV vaccination is not completely effective, it did provide partial protection which modified the time course of the infection. The PRRSV vaccination effect was also modeled as a time dependent process which was additively applied to the disease equation. The model predicted intake and growth depressions for both pathogens and the effect of vaccination with minimal mean and slope bias indicating the model represented the data well. Surprisingly the salmonella equation also did well in describing the negative effects of an e-coli infection suggesting that it could be used to predict the effects of other digestive pathogens. The reductions in feed intake explained all of the changes in animal performance, and thus no additional equations were required to simulate potential decreases in diet digestibility or increases in animal maintenance requirements.
The modified model was incorporated into the grower submodel of the overall barn model to allow simulations of PRRSV and salmonella infections and PRRSV vaccination.
The Swine Environmental Research Building (SERB)
The Swine Environmental Research Building (SERB) is set up at a scale that can validate the results of pilot scale studies done elsewhere. It houses 720 pigs in 12 rooms with 6 pens per room and 10 pigs per pen. Manure is quantitatively collected and stored in a deep pit under each side of the room (3 pens of 10 pigs each). The two manure pits in each room are divided by a wall under the central walkway. The building is equipped with a centralized laboratory capable of monitoring GHG emissions from each independently ventilated room. Pigs will be supplied by Purdue or obtained from a commercial source at weaning, blocked by weight and sex and randomly assigned to treatments.
Dr. Radcliffe discussed his efforts at SERB during a webinar on Life Cycle Assessment Modeling in the Pork Industry.
Additional Projects Related to Improving the Environmental Footprint of Pork Production in the U.S.
Acknowledgements
Dr. Scott Radcliffe
jradclif@purdue.edu
Phone: (765) 496-7718
Dr. Mark Hanigan
mhanigan@vt.edu
Phone: (540) 231-0967
Dr. Charles Maxwell
University of Arkansas (retired)
This information is part of the program “Integrated Resource Management Tool to Mitigate the Carbon Footprint of Swine Produced In the U.S.,” and is supported by Agriculture and Food Research Initiative Competitive Grant no. 2011-68002-30208 from the USDA National Institute of Food and Agriculture. Project website: https://lpelc.org/integrated-resource-management-tool-to-mitigate-the-carbon-footprint-of-swine-produced-in-the-united-states/.

