Purpose
Dairy farms employing flushing systems often encounter significant challenges in managing substantial volumes of recycled water, which can have environmental, economic, and operational implications. This study aims to evaluate a multi-stage process designed to improve solid/nutrient extraction from flushed water already treated by a pull-plug sediment basin system.
What Did We Do?
We implemented a three-stage sequential treatment process comprising coagulation, Fenton oxidation, and membrane filtration. In the first stage, coagulation was performed using aluminum sulfate (Al₂(SO₄)₃) to remove colloidal solids from the treated barn flushing water. The optimal alum dosage (500–7,000 mg/L) was determined based on turbidity, total solids, and chemical oxygen demand (COD) removal.
The second stage involved Fenton oxidation, where hydroxyl radicals generated from hydrogen peroxide (H₂O₂) and an iron catalyst (Fe²⁺) further degraded organic pollutants. Utilizing response surface methodology (RSM), we optimized the concentrations of H₂O₂ (500–1,800 mg/L), FeCl₃ (250–950 mg/L), and reaction time (15–50 min) to achieve a balance between treatment effectiveness and cost efficiency.
In the final stage, ultrafiltration and reverse osmosis were employed to remove dissolved ions, ensuring compliance with discharge standards.
What Have We Learned?

The results indicated that turbidity removal peaked at a dosage of 5,000 mg/L of Al₂(SO₄)₃, while total solids and COD removal stabilized at 4,000 and 5,000 mg/L, respectively. Although turbidity initially increased following the coagulant addition, the formation of aluminum hydroxide flocs facilitated effective pollutant removal. To balance reagent costs and treatment efficiency, a dosage of 4,000 mg/L alum was selected. After coagulation, the coagulated supernatant underwent fenton oxidation.
Turbidity removal (%)
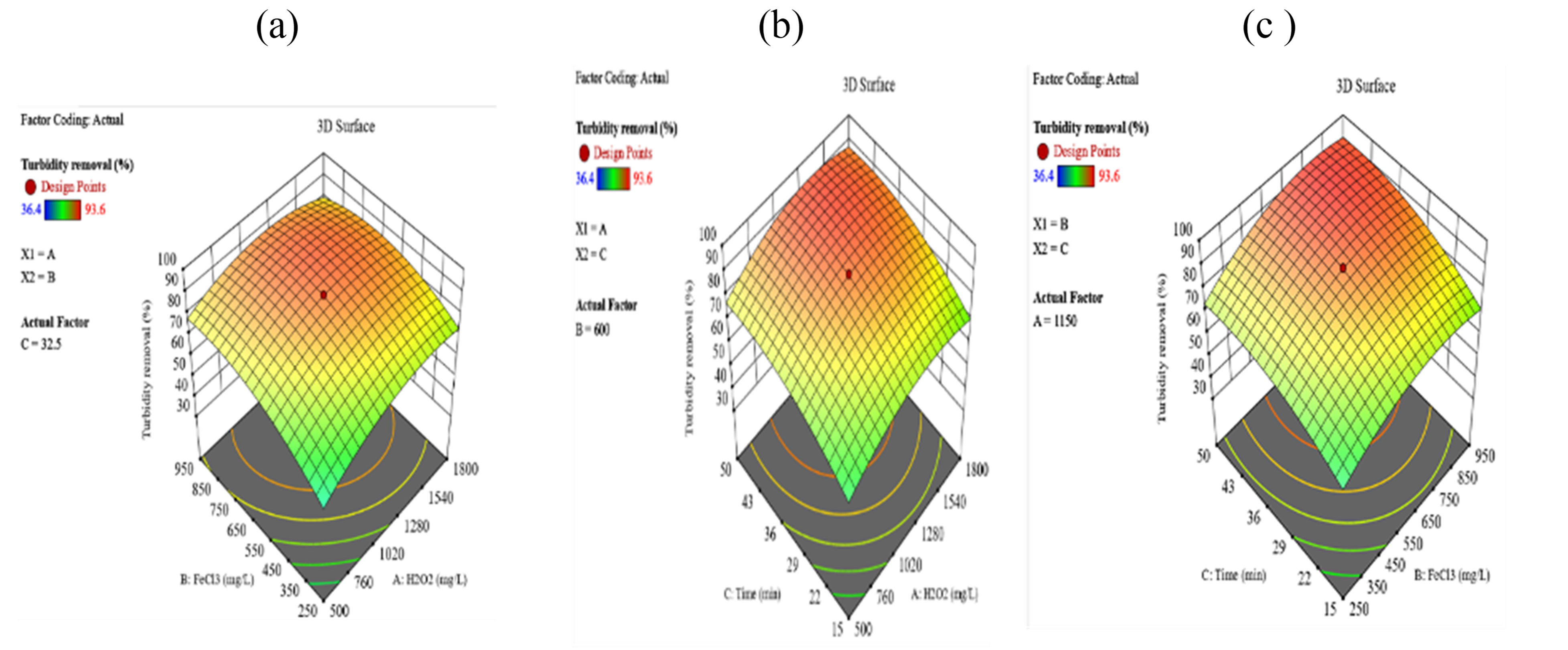
Response surface analysis confirmed that optimal turbidity removal was achieved with H₂O₂ concentrations of 1,280-1,800 mg/L and FeCl₃ concentrations of 550-950 mg/L. Furthermore, a minimum mixing of 36 minutes was necessary to attain maximum efficiency.
Total solid removal (%)

For total solids removal, effective interaction was observed at H₂O₂ levels of 500–1,240 mg/L and FeCl₃ concentrations of 250–450 mg/L. Mixing times exceeding 43 minutes were found to reduce removal efficiency.
COD removal (%)
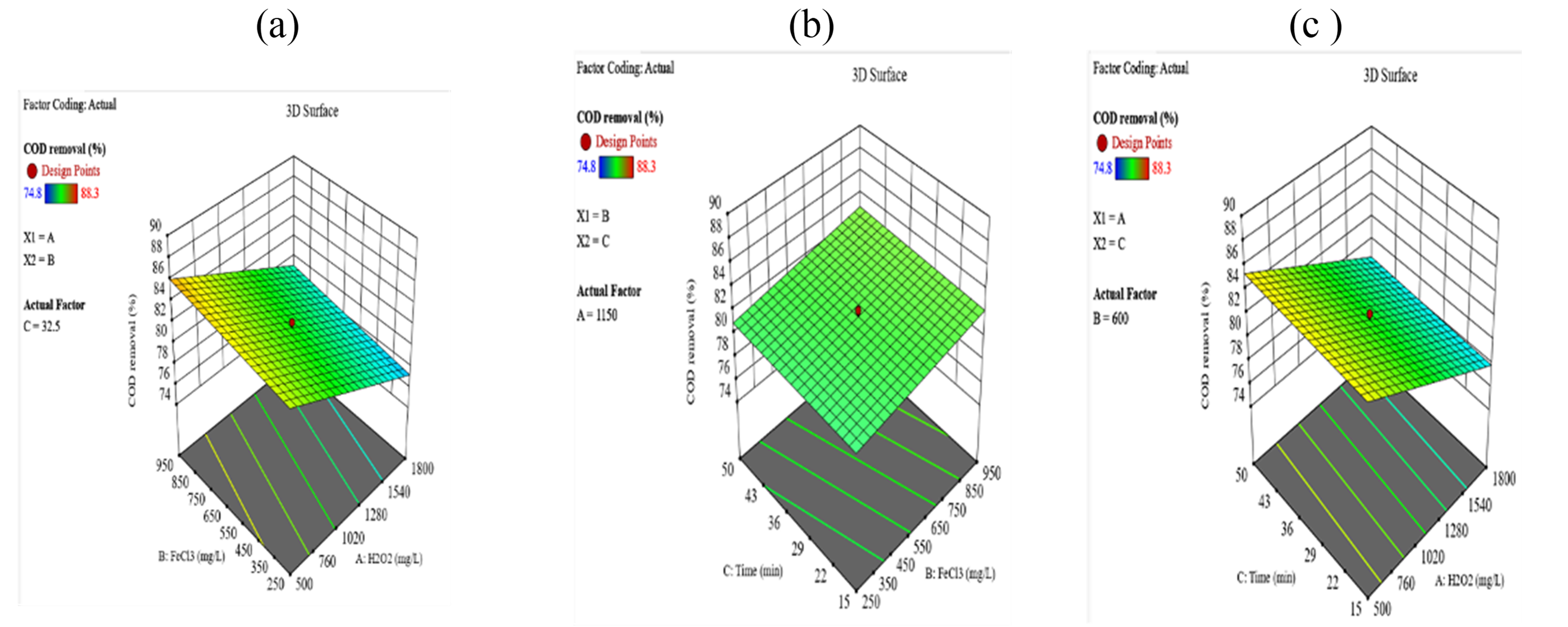
COD removal was most effective within the H₂O₂ range of 500–760 mg/L and FeCl₃ concentrations of 450–950 mg/L, while mixing time had minimal impact.
Cost ($)
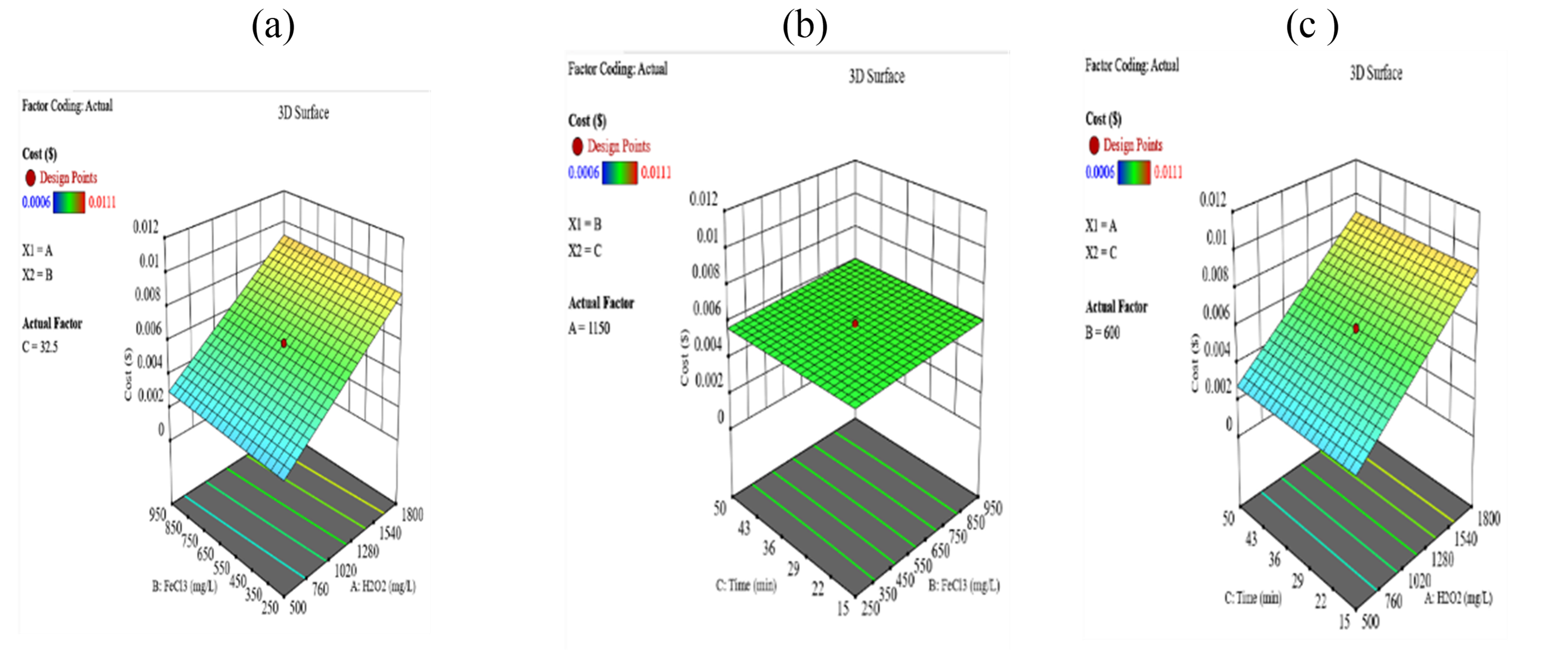
Regarding treatment cost, H₂O₂ was identified as the most influential cost factor due to its higher price. To balance removal efficiency and cost, the optimized conditions were determined as 563.3 mg/L H₂O₂, 568.4 mg/L FeCl₃, and a 33-minute reaction time, according to the calculations of RSM model. This setup achieved 86.4% turbidity removal, 18.7% total solids removal, and 81.5% COD removal at a treatment cost of $0.03 per liter of wastewater.
Future Plans
The next phase of the study will focus on membrane filtration experiments to further remove dissolved ions and ensure compliance with discharge standards. Additionally, a systematic economic analysis will assess cost-effectiveness, scalability, and operational feasibility for large-scale dairy farm applications.
Authors
Presenting author
Moh Moh Thant Zin, Post-doctoral researcher, University of Missouri-Columbia
Corresponding author
Teng-Teeh Lim, Extension Professor, University of Missouri-Columbia, limt@missouri.edu
Acknowledgements
Funding is provided by USDA-NIFA, grant award (2018-68011-28691) and University of Missouri Extension.
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2025. Title of presentation. Waste to Worth. Boise, ID. April 7-11, 2025. URL of this page. Accessed on: today’s date.

