![]() Waste to Worth home | More proceedings….
Waste to Worth home | More proceedings….
Abstract
A study was conducted to evaluate the pathogen inactivation on 9 dairy facilities in Wisconsin with a combination of anaerobic digestion and solid/liquid separation technologies. Samples were collected every 2 weeks over the course of eight months to assess dairy pathogen inactivation in full-scale operational digesters and solid/liquid separators. Samples were then analyzed by qPCR for pathogens including protozoa, bacteria. bovine viruses, and indicators.
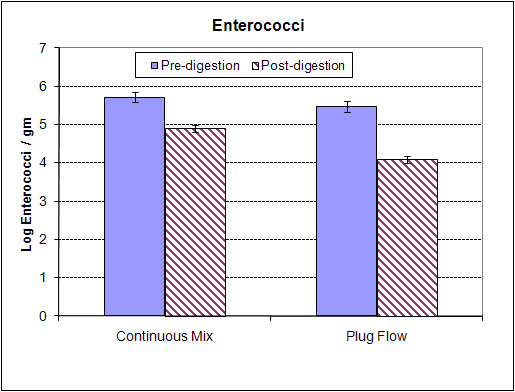
Preliminary results indicate full-scale anaerobic digesters reduce pathogen levels by 99% to 99.9%. And after digestion and separation of the digestate, the liquid fraction contains the majority of pathogens. Although the solids fraction contained fewer pathogens, the concentration could still be above the infectious dose, particularly for calves. Results have implications for a variety of digestate end uses including bedding and land spreading.
Purpose
Anaerobic digestion and bedding recovery units are increasing in on-farm use around the United States as a component of manure management systems. Nearly all on-farm systems with a digester in the United States have a mechanical solid/liquid separation system following digestion which fractions the digestate into a solid and a liquid product. Processing of manure using digestion and/or a solid/liquid separation process can impact the nutrient and pathogen content of each stream. Lack of data for real world performance has limited the use of end products and has reduced revenues and resulted in operational problems for many dairies in Wisconsin.
The purpose of this study was to evaluate the fate of pathogens and nutrients through full scale anaerobic digestion and solid liquid separation systems to better understand the impacts of manure processing.
What Did We Do?
In order to assess real world performance of digesters and solid/liquid separation systems, an assessment of 9 on-farm systems was conducted over the course of one year. The study design includes sampling every other week pre and post digestion (if a digester is on-farm) and the solid and liquid portion after separation. This allows for assessment of the digestion process and the separation system. Samples are evaluated for nutrients, solids, pathogens (particularly those associated with herd health) and pathogen indicators. The results indicate impacts to pathogen and nutrient concentrations throughout the system.
What Have We Learned?
Pathogen content from farm to farm and within one farm varies significantly. Performance of digesters on pathogen destruction is extremely variable. Through the solid/liquid separation process the majority of the pathogens within the stream remain in the liquid portion.
Future Plans
To continue evaluation through controlled systems to identify key operational techniques to increase pathogen removal.
Authors
Rebecca Larson, Assistant Professor, University of Wisconsin – Madison, ralarson2@wisc.edu
Mark Borchardt, Research Microbiologist, USDA – ARS
Asli Ozkaynak, Post-Doctoral Researcher, University of Wisconsin – Madison
Susan Spencer, Research Microbiologist, USDA – ARS
Additional Information
Data is to be published
Acknowledgements
Funded by the USDA
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2013. Title of presentation. Waste to Worth: Spreading Science and Solutions. Denver, CO. April 1-5, 2013. URL of this page. Accessed on: today’s date.