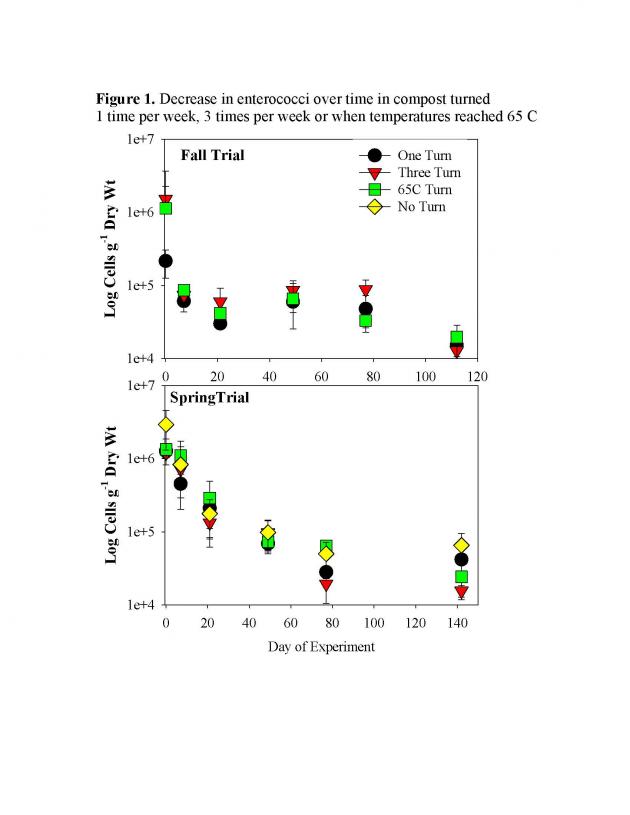
Escherichia coli transported in surface runoff from dissolution of applied poultry litter is a major variable in assessing fecal contamination of streams. However, the relative magnitude of the E. coli concentration from a specific poultry litter application and relative to the time lag between litter application and rainfall are not completely understood. This research investigated E. coli transport in runoff on fourteen 2 m × 2 m pastureland plots. Poultry litter was manually applied (4,942 kg ha‐1) in twelve plots followed by artificial rainfall with intensities equivalent to 2‐year and 5‐year storm events. Rainfall was applied in duplicate plots immediately after poultry litter application and 24 and 120 h after litter application. Experiments were also conducted on two control plots without poultry litter application. Surface runoff was collected using a flume installed in a trench. E. coli was quantified from sampled runoff and used as an indicator of fecal contamination by the most probable number (MPN) technique. Significant differences in the average event mean concentrations (EMCs) for the various treatments were determined using ANOVA. No significant differences were observed in average EMCs relative to storm intensity. Statistically significant differences were observed in average EMCs relative to time lag between litter application and rainfall (P < 0.05). A nonlinear relationship was observed between average E. coli EMC and time lag, with the EMC decreasing between 0 h (1.6 × 105 MPN/100 mL) and 24 h (1.3 × 104 MPN/100 mL) and then increasing at 120 h (4.3 × 104 MPN/100 mL). E. coli were always detected in the control plots (average EMC of 6.8 × 103 MPN/100 mL), indicating the presence and transport of fecal bacteria from sources independent of the immediate poultry litter application. Even though poultry litter application may increase E. coli concentrations in runoff, other sources of fecal contamination serve as a significant component of the total E. coli EMC, especially as the time lag between litter application and rainfall events increases.
Purpose
Poultry litter is recognized as an excellent source of the plant nutrients nitrogen, phosphorus and potassium. In addition, litter returns organic matter and other nutrients such as calcium, magnesium and sulphur to the soil, building soil fertility and quality.
Questions exist concerning E. coli contamination of waterways following manure land application events. Oklahoma State University researchers conducted a field study evaluating surface runoff transport of E. coli following poultry litter application to pastureland.
 What did we do?
What did we do?
Pasture plots, which consisted of ryegrass, fescue grass, bermudagrass and some Johnsongrass, were established at the Eastern Oklahoma Research Station located in Haskell, OK. Cattle had not been allowed access to the pasture for over one year and poultry litter had previously been applied one year prior to the study. Broiler litter was applied to 14 plots at a rate of 2.2 tons/acre. Two control plots received no litter application.
An artificial rainfall simulator was used to produce 2 yr and 5 yr storm events. Rainfall was applied at 0 h, 24 h and 120 h after litter application. Surface runoff was collected using a flume installed in a trench (Figures 1 and 2). Water samples were tested for E. coli populations.

What have we learned?
Results of this study showed that E. coli event mean concentrations (EMC) in sampled runoff decreased at 24 h and 120 h when compared to 0 h after litter application (Table 1). However, a slight increase in populations was observed at 120 h as compared to 24 h. This slight growth may have been due to litter in contact with the soil surface and protected from ultraviolet light and moisture loss by vegetative cover.
In control plots, E. coli was always detected, indicating other sources of E.coli aside from poultry litter. Other sources may include rodents, birds, and other small mammals.

In conclusion, poultry litter applications may contribute to runoff of E. coli when rainfall events occur shortly after litter application. However, other sources of fecal contamination may serve as a significant component of the total E. coli EMC, especially as the time lag between litter application and rainfall event increases. The implications of this study may affect poultry litter application timing decisions based on predicted rainfall events.
Future Plans
Future studies using more advanced biological analysis techniques (i.e., DNA profiling) should be conducted to identify sources of background E. coli concentrations.
Authors
Josh Payne, Area Animal Waste Managment Specialist, Oklahoma State University joshua.payne@okstate.edu
Jorge Guzman, Senior Engineer, Waterborne Environmental; Garey Fox, Professor, Oklahoma State University
Additional information
Guzman, J. A., G. A. Fox and J. B. Payne, 2010. Surface runoff transport of Escherichia coli after poultry litter application on pastureland. Trans. ASABE. 53(3):779-886.
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2015. Title of presentation. Waste to Worth: Spreading Science and Solutions. Seattle, WA. March 31-April 3, 2015. URL of this page. Accessed on: today’s date.