Proceedings Home | W2W Home
Purpose
A major source of pollution and loss of nutrient value from animal manure results from the conversion of urea nitrogen into ammonia by the naturally occurring urease enzyme in solid/liquid waste streams. Studies often focus on either urease inhibition in soil to prevent the volatilization of applied urea fertilizer or recovery of ammonia from wastewater, but few have studied urease inhibition in manure slurry directly from the barn. If the urea in fresh urine can be preserved at the source it would prevent the volatilization of ammonia that represents the loss of a valuable nutrient as well as the adverse effects of ammonia on livestock, humans and the environment. Our study investigated methods of inhibiting urease activity in fresh swine urine to preserve the urea nitrogen content during storage, processing and transport.
What did we do?
The study was comprised of 4 experiments:
1) Jack bean urease was introduced to a 1M aqueous urea solution and fresh swine urine. Samples were taken hourly for five hours and lab tested for total ammoniacal nitrogen (TAN) to compare urease activity of the urea solution with that of actual urine.
2) Using the same 1M urea solution, the effects of pH < 3.0 and pH > 12.0 on urease activity was measured relative to the commercially available inhibitors N-(n-butyl) thiophosphoric triamide (NBPT), salicylhydroxamic acid (SHAM), and Thymol (a phenol obtained from thyme oil or other volatile oils). Each treatment was sampled weekly for Total Kjeldahl Nitrogen (TKN), TAN and pH over six weeks to see which treatment best preserved urea nitrogen.
3) To determine if a smaller pH adjustment would be an effective inhibitor, we compared the activity of urease in a 1M urea solution across a pH range from 4.0 to 11.0. This was done by either lowering the pH of the urea solution with 0.1N sulfuric acid or raising it with 0.5N sodium hydroxide. The samples were tested at 7 days for pH, TKN and TAN.
4) Finally, we explored the effect of pH < 3.0 and pH > 12.0 on urease activity in swine urine to compare the effect with that in the urea solution. The initial pH, TKN and TAN of the swine urine was observed relative to the pH and concentrations of samples taken at 7 days and 14 days.
What have we learned?
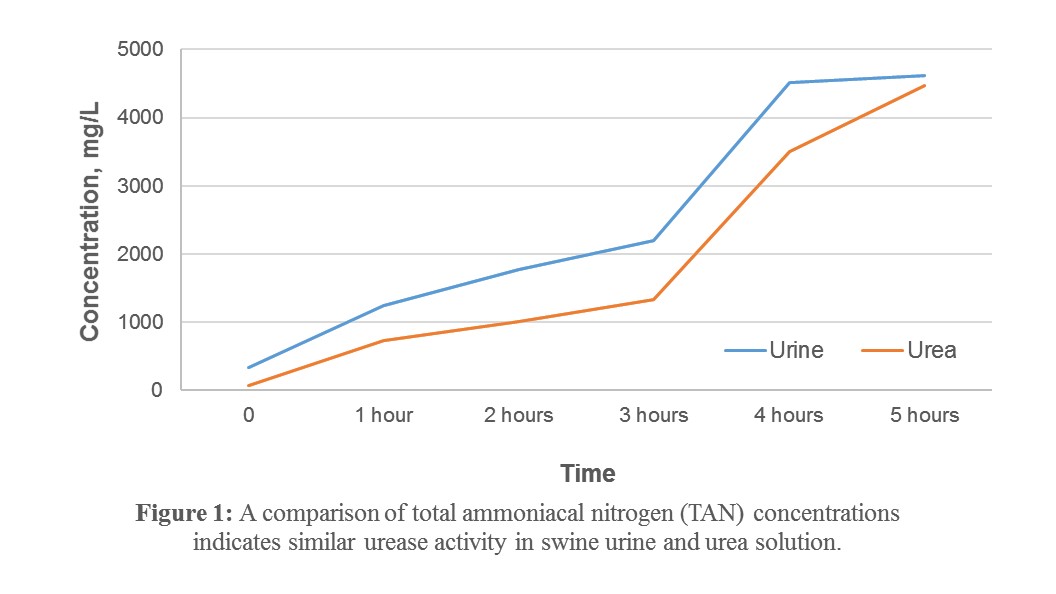
1) The conversion of urea nitrogen to ammonia (as measured by TAN) follows a similar trend in both a urea solution and freshly collected sow urine (Figure 1). This indicates that a urea solution may be an acceptable alternative for testing urease inhibition when fresh urine is not available.
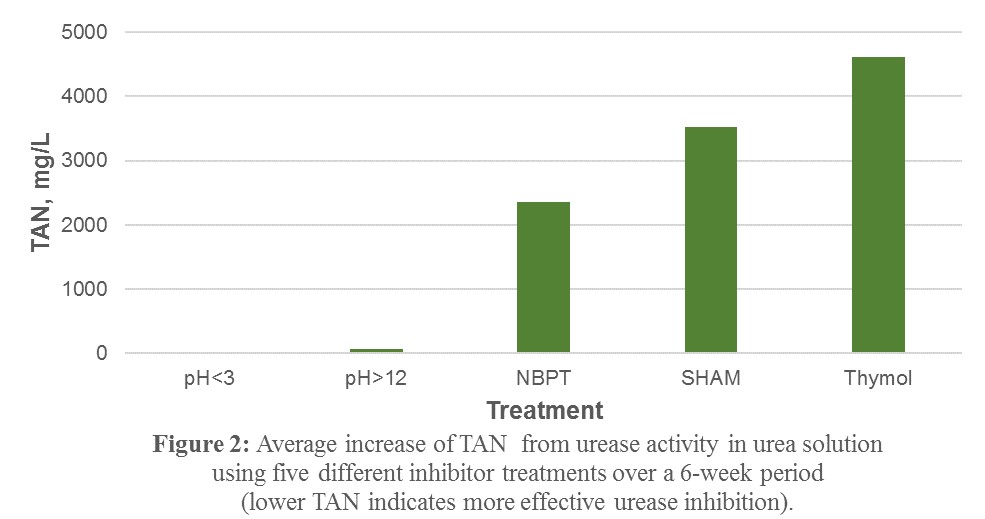
2) In a comparison of NBPT, Thymol, and SHAM to pH < 3.0 and pH > 12.0, it was observed that the high and low pH had the most significant inhibitory effect on urease enzyme activity, as almost none of the TKN in the samples observed over a 6-week study period was converted to TAN, relative to the other inhibitors tested (Figure 2).
3) Testing a range of nominal pH values between 4.0 and 11.0 it was observed that while urease enzyme remained active over a 2 week period across all values, activity declined with an increase or decrease in pH from the highest activity observed at pH 7.0. However, at a pH below 3.0 the urease enzyme was completely denatured and could not be restored by increasing the pH.
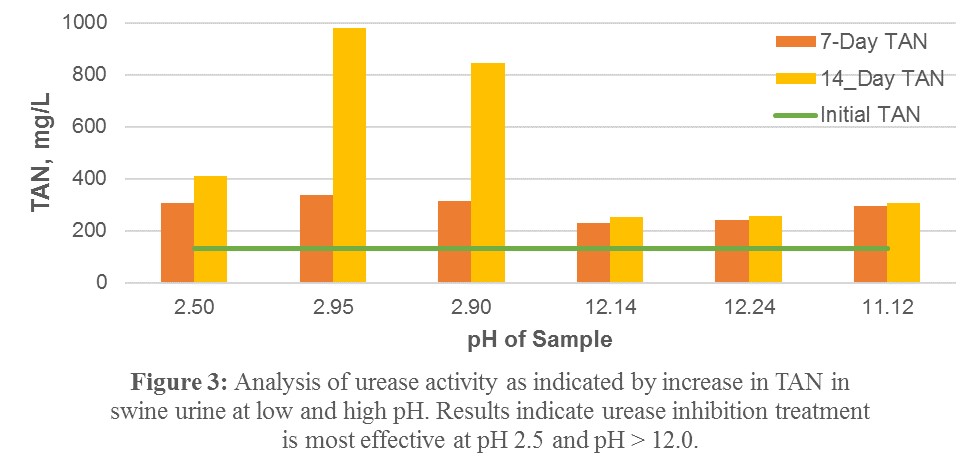
4) When testing high and low pH on swine urine it was observed to have a similar inhibitory effect on urease activity compared with the urea solution, that the effect is lasting over 14 days, and that the high pH is slightly more effective than the low pH (Figure 3).
Future Plans
A follow up study will be conducted using a pilot scale scraper separation system to collect fresh urine from about 30 swine through a 16 week growing cycle. We will be testing urea preservation using 3 different inhibitor treatments including pH > 12, pH < 3 and the commercial soil urease inhibitor, NBPT. We will also study the effect of UV light on urease activity during the control periods. The experiment will be repeated for each inhibitor over 3 feeding phases to simulate grower farm conditions.
Corresponding author, title, and affiliation
Alison Deviney, Graduate Research Assistant at Biological and Agricultural Engineering Department, North Carolina State University
Corresponding author email
Other authors
John J. Classen, Ph.D. and Mark Rice, Extension Specialist at Biological and Agricultural Engineering Department, North Carolina State University
Additional information
Alison Deviney
Biological and Agricultural Engineering Department
North Carolina State University
Raleigh, NC 27695
Acknowledgements
Jason Shye and Dan Wegerif, Managing Members
Waste 2 Green, LLC, Cocoa, Florida, USA

