Purpose
The main objective of this research was to investigate the electrochemical oxidation of swine manure effluent obtained from a primary lagoon for reducing organic and inorganic pollutants.
What did we do?
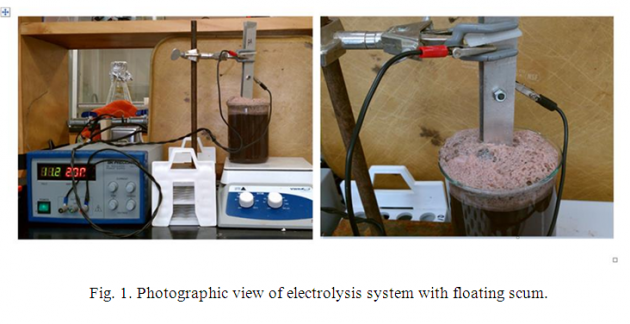
Liquid swine manure effluent was collected and treated with three different electrodes (Fe-Fe, Al-Al, and Fe-Al) with three electric current levels (500, 1000, and 2000 mA). The electrochemical cell consisted of two parallel rectangular plates (90 mm × 25 mm × 1.5 mm) of iron (Fe-Fe), aluminum (Al-Al), and iron-aluminum (Fe-Al, later on described as hybrid) electrodes; immersed in a beaker with 550 mL swine effluents, and powered by a direct current (DC) supply (Figure 1). All studies were conducted in batches at room temperature.
What have we learned?
In general, removal efficiencies increased with increasing current densities and electrolysis times for electrodes evaluated. Aluminum electrodes (Al-Al) outperformed iron (Fe-Fe) and hybrid electrodes (Al-Fe) in removing total phosphorus (TP) at all current density levels tested. At higher current density (21 mA cm-2), Fe-Al electrodes had shown better TP removal efficiency than other electrodes within 1200 s of treatment initiation, but at lower current densities (5 and 10 mA cm-2) Al-Al electrodes outperformed the other electrodes for TP removal. TP removal progressed overtime and maximum TP removal occurred within 1800 s from the start of each experiment. Overall, Al-Al electrodes resulted in better TP removal over all current densities during the experimental period. Overall, use of hybrid electrodes resulted in better chemical oxygen demand (COD) removal. For the same treatment times (1200 s) at higher current density (21 mA cm-2), hybri d electrodes removed about 100% COD, which are about 1.9 and 1.3 times higher than those of aluminum and iron electrodes, respectively. Iron electrodes showed the highest removal efficiency (85%) for total organic carbon (TOC) at 21 mA cm-2 current density and 1200 s treatment time.
In this study, Fe-Fe electrodes at 10 and 21 mA cm-2 current densities, removed higher TOC (17 to 85% and 51 to 100%, respectively) than those of Fe-Al (9 to 73% and 44 to 84%, respectively) and Al-Al electrodes (9 to 67% and 14 to 70%, respectively). On the contrary, Al-Al electrodes removed higher TOC (10 to 69%) than those of Fe-Fe and Fe-Al electrodes at lower current density (5 mA cm-2) for all treatment times (600 to 3600s). The performance of Fe-Fe and Fe-Al electrodes at 5 mA cm-2 current density were close (~ 6 to 50%) for all treatment times. The high removal efficiency for TOC (85%) was achieved at 1800 s treatment time at 21 mA cm-2 current density with Fe-Fe electrodes.
The removal efficiencies of TP, COD, and TOC were positively correlated with increasing treatment times and three levels of current densities (5, 10, and 21 mA cm-2) and treatment times for each electrode type. Removal efficiencies of TP for all three electrodes (Fe-Fe, Fe-Al, and Al-Al) were similar (90 to 100%) at 21 mA cm-2 current densities for a 1200 s treatment time. Removal efficiencies of TP during 1800 s treatment time for all three electrodes at 21 mA cm-2 current density were about 100%. The TP removal efficiency with Al-Al electrodes was about 100% at 1800 s treatment time for all three levels of applied current densities. Even at lower current density (5 mA cm-2), removal efficiencies of TP with Al-Al electrodes was higher than those of Fe-Fe and Fe-Al electrodes.
Overall, lower specific electrical energy consumptions (SEECs) per kg of pollutants (TP, COD, and TOC) were estimated for the aluminum electrodes than the other electrodes combination. Based on this lab study, it can be concluded that depending on the target pollutants, an appropriate current density and electrode type may be applied to obtain the maximal removal of a pollutant.
Future Plans
This study was a lab scale study. In the future, a pilot scale study will be conducted with different additives and electrode types.
Authors
Shafiqur Rahman, Associate Professor, Agricultural and Biosystems Engineering, North Dakota State University Agricultural and Biosystems Engineering, North Dakota State University s.rahman@ndsu.edu
M. S. Borhan, Research Specialist, Agricultural and Biosystems Engineering, North Dakota State University
Additional information
http://thescipub.com/abstract/10.3844/ajabssp.2014.490.502
Acknowledgements
Financial support from North Dakota Pork Council and State Board of Agricultural Research and Education (SBARE)are gratefully acknowledged. The authors also acknowledge Swine Research Center of North Dakota State University for supplying swine effluents for this study.
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2015. Title of presentation. Waste to Worth: Spreading Science and Solutions. Seattle, WA. March 31-April 3, 2015. URL of this page. Accessed on: today’s date.

