Purpose
The purpose of this project was to demonstrate a mobile fluidized-bed cone for extraction of phosphorus in the form of struvite (magnesium-ammonium phosphate) from undigested (raw) liquid dairy manure. Since Ca binds inorganic P, a particular emphasis was placed on evaluating the effect of oxalic acid as an acidifier and Ca binder.
Dairies often have excess P in manure in relation to the need for on-farm crop production. Easily mineable reserves of phosphorus (P) worldwide are limited, with a majority residing in Morocco (USGS 2013). One approach to recycling P is to capture excess P from dairy manure in the form of struvite for off-farm export as a nutrient source for crop production.
What we did
A portable trailer-mounted fluidized-bed cone (volume of 3200 L) was used to extract phosphorus in the form of struvite (magnesium-ammonium phosphate) from undigested (raw) liquid dairy manure. Batches of 13,000 liters of manure were evaluated and the system was operated at a flow rate of ~ 32 liters per minute. Sulfuric acid or oxalic acid-sulfuric acid were used to decrease the pH, and sodium hydroxide was used to raise the pH. Oxalic acid was chosen for evaluation due to its dual ability to decrease pH and bind calcium.
What we learned
Results of concentration of total P or ortho-P (OP) after manure treatment through the fluidized bed suggested no advantage of the combination of oxalic acid with sulfuric acid to decrease the concentration of P (see Figures 1 and 2). More detailed analyses of centrifuged post-bed samples of manure effluent indicated that the oxalic acid was binding the free calcium, but the resulting Ca compounds remained suspended in the effluent. Centrifuged manure samples had Ca contents ~23% of un-centrifuged samples when oxalic/sulfuric acid was used as a pH reducer. Centrifuged manure samples had Ca contents ~84% of un-centrifuged samples when sulfuric acid was used as a pH reducer. With raw manure, oxalate does not appear to be beneficial, unless there is a more effective step to drop Ca-oxalate out of suspension, such as centrifuging.
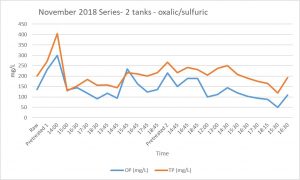
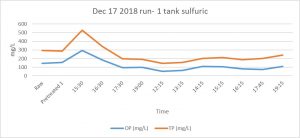
Future Plans
Anaerobically digested (AD) manure will be evaluated with the same set of conditions that were utilized with raw dairy manure to determine potential benefits of using oxalic acid with AD manure.
Authors
Joe Harrison1, Kevin Fullerton1, Elizabeth Whitefield1, and Keith Bowers2.
1Washington State University
2Multiform Harvest
jhharrison@wsu.edu
Citations and video links
U.S. Geological Survey, Mineral Commodity Summaries, January 2013. http://minerals.usgs.gov/minerals/pubs/commodity/phosphate_rock/mcs-2013-phosp.pdf
The Mobile Struvite Project Overview Video: Capturing Phosphorus from Liquid Dairy Manure and Cost Efficient Nutrient Transport
Dairy Struvite Video: Capturing Phosphorus from Dairy Manure in the Form of Struvite on 30 Dairy Farms in WA state
Alfalfa Struvite Video: Struvite, a Recycled Form of Phosphorus from Dairy Manure, used as Fertilizer for Alfalfa Production
Acknowledgements
This project funded by the USDA NRCS CIG program and the Dairy Farmers of Washington.
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2019. Title of presentation. Waste to Worth. Minneapolis, MN. April 22-26, 2019. URL of this page. Accessed on: today’s date.

