Waste to Worth home | More proceedings….
Abstract
An economical process to capture residual ammonia nitrogen and reduce the production of new ammonia via the Haber process is needed. The CO2, N2O and NOx emissions from nitrification and denitrification of industrially created ammonia will be reduced as a result. The ammonia product should be sold at a profit, but less than $1,700 / ton N.
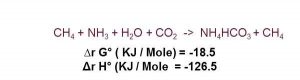
This paper describes the ABC process and presents the ammonia recovery and biomethane production results of a pilot investigation of the ABC process for the recovery of ammonia nitrogen. The work was supported by the US Department of Agriculture (USDA) under a Small Business Innovative Research project. The ABC process uses no chemicals and very little energy. The process recovers the ammonia as crystalline ammonium bicarbonate (ABC). In the process of producing the ABC, carbon dioxide is removed from the biogas to produce “biomethane”, a transportation quality fuel at little or no cost.
|
|
Is It Possible to Recover Ammonia Economically?
The discharge of ammonia nitrogen is a well recognized adverse consequence of anaerobic waste treatment. As a result, further treatment to remove ammonia is required. A wide variety of processes have been developed to address the “ammonia issue”. The commonly used processes are the many variations of nitrification / denitrification and Anammox processes. The Anammox (anaerobic ammonium oxidation) process is the least expensive and produces significantly less GHG (N2O). The nitrification / denitrification and Anammox processes directly convert ammonia to nitrogen gas (N2) resulting in the loss of the ammonia resource at a treatment cost of approximately $1,600 / ton N for a large facility. The ammonia that is destroyed must be replenished through the Haber-Bosch process that requires 32 GJ of energy per ton of ammonia to produce and similar energy consumption to transport. The production and transport have a cost of $1,200 / ton N while producing substantial GHG emissions. The minimum total cost of destroying and replacing ammonia is greater than $2,800 / ton N. An economical process to capture residual ammonia nitrogen for reuse, while reducing the production of new ammonia via the Haber process, is needed. The CO2, N2O, and NOx emissions from nitrification and denitrification of industrially created ammonia will be reduced as a result.
A number of processes have been developed over the past 50 years to remove and recover ammonia as an ammonium sulfate or nitrate fertilizer. Several facilities were constructed in the EU in the 1970’s. Those facilities were however uneconomical because of the high cost of chemicals (acid, lime, sodium hydroxide) and sludge disposal. Modification of those processes that use ion exchange, as opposed to ammonia stripping, remain uneconomical since they also require caustic, salt, and sulphuric acid to remove ammonia and recover ammonium sulphate. An economical process that can recover ammonia as a solid product without the use of hazardous chemicals is required.

Figure 2 Ammonium Bicarbonate (ABC) |
What Did We Do?
E3 developed the Ammonium Bicarbonate Recovery (ABCR) process that recovers the ammonia as a crystalline solid pathogen free, inorganic fertilizer without the use of any chemicals. In the process of producing the Ammonium Bicarbonate (ABC), carbon dioxide is removed from the biogas to produce “biomethane”, a transportation quality fuel at little or no cost. The products of the process are biomethane quality transportation fuel and solid ammonium bicarbonate fertilizer that can be used for the pretreatment of lignocellulosic substrates.
To overcome the ammonia reclamation process deficiencies, E3 developed the Rotating Photo Bioreactor (RPB) shown in Figure 1. The RPB is a horizontal ammonia stripping reactor that removes the ammonia without the use of any chemicals. An operating demonstration can be seen here
The stripped ammonia and water vapor are condensed to form a concentrated aqua ammonia solution. Turbid, ammonia laden, anaerobic digestate flows through a fixed film photo bioreactor, that uses natural and/or artificial light, to culture cyanobacteria that consume the bicarbonate in the digestate thus raising the pH to values exceeding 10. At the higher pH, the ionized ammonia (NH4+) is shifted to the gas form, NH3 that can be stripped by the low pressure gas flowing from the condensation unit over the upper portion of the rotating disks. Very little blower pressure is required. The impact of digestate turbidity is minimized by the thin liquid film flowing over the partially submerged rotating disks supporting the bicarbonate consuming cyanobacteria that require light. The ammonia laden gas is then returned to the condenser where the ammonia gas and water are condensed to recover concentrated aqua ammonia. The system operates at low liquid and gas pressures through the use of a heat pump and low pressure gas blower.
 |
The aqua ammonia condensate is recovered when the effluent is being discharged from the digester. The condensate is stored in a tank for use throughout the day to clean the biogas by removing the carbon dioxide and hydrogen sulfide in the digester’s gas. The ammonia condensate is sprayed into the biogas stream where the ammonia and water react with the carbon dioxide to produce a solid ammonium bicarbonate precipitate that is removed, bagged, and stored as a renewable, low carbon footprint fertilizer to be applied to the fields when needed for crop growth or blended with the solid residuals to produce a balanced fertilizer.
What Have We Learned?
The pilot investigation substantiated that high BTU (990±) biomethane could be produced from biogas while recovering 85%± of the ammonia present in the digestate at less capital and O&M cost of producing electricity.
Future Plans
The current plan is to build a full scale operating facility treating high nitrogen content manure such as poultry manure.
Author
Dennis A. Burke, CEO, Environmental Energy & Engineering Company engineer@makingenergy.com
The authors are solely responsible for the content of these proceedings. The technical information does not necessarily reflect the official position of the sponsoring agencies or institutions represented by planning committee members, and inclusion and distribution herein does not constitute an endorsement of views expressed by the same. Printed materials included herein are not refereed publications. Citations should appear as follows. EXAMPLE: Authors. 2013. Title of presentation. Waste to Worth: Spreading Science and Solutions. Denver, CO. April 1-5, 2013. URL of this page. Accessed on: today’s date.


